Question: Calculate the enthalpy change, H, for the process in which 21.6g of water is converted from liquid at 19.6C to vapor at 25.0C. For water,
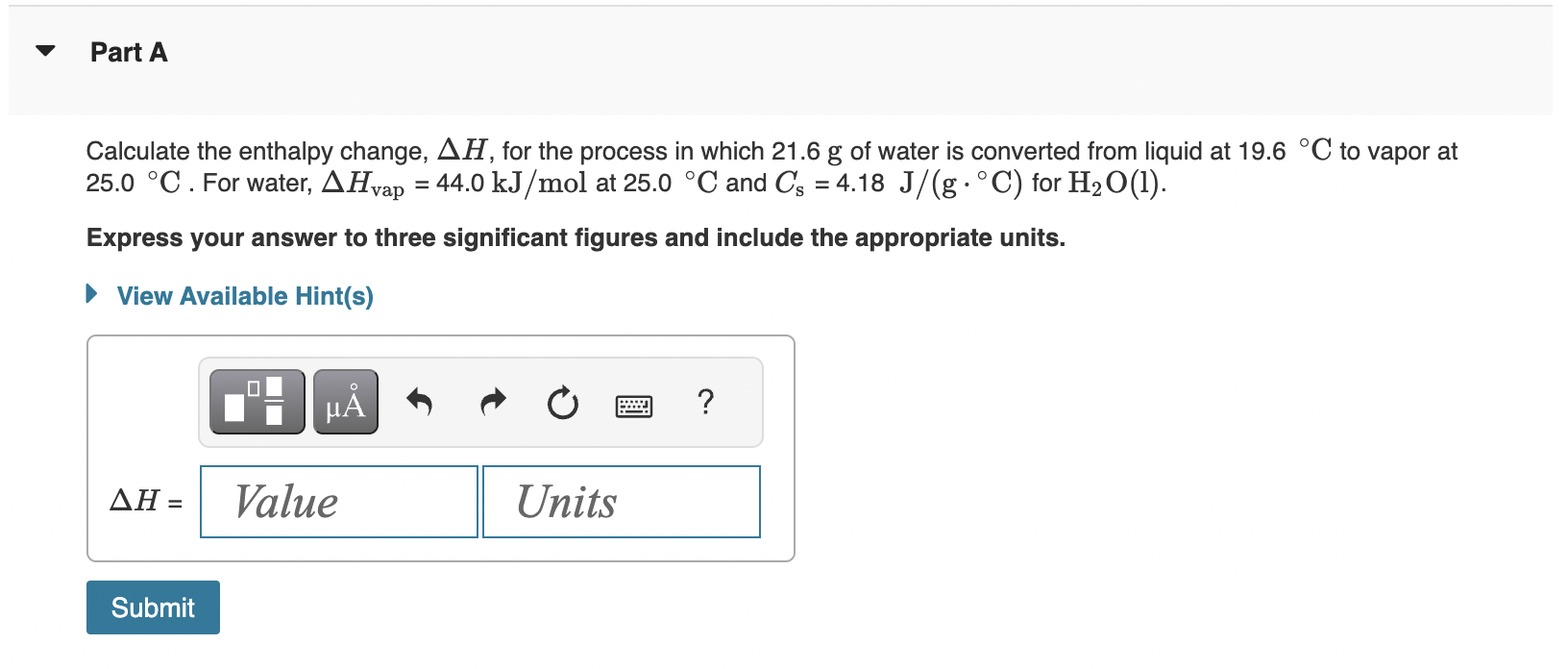
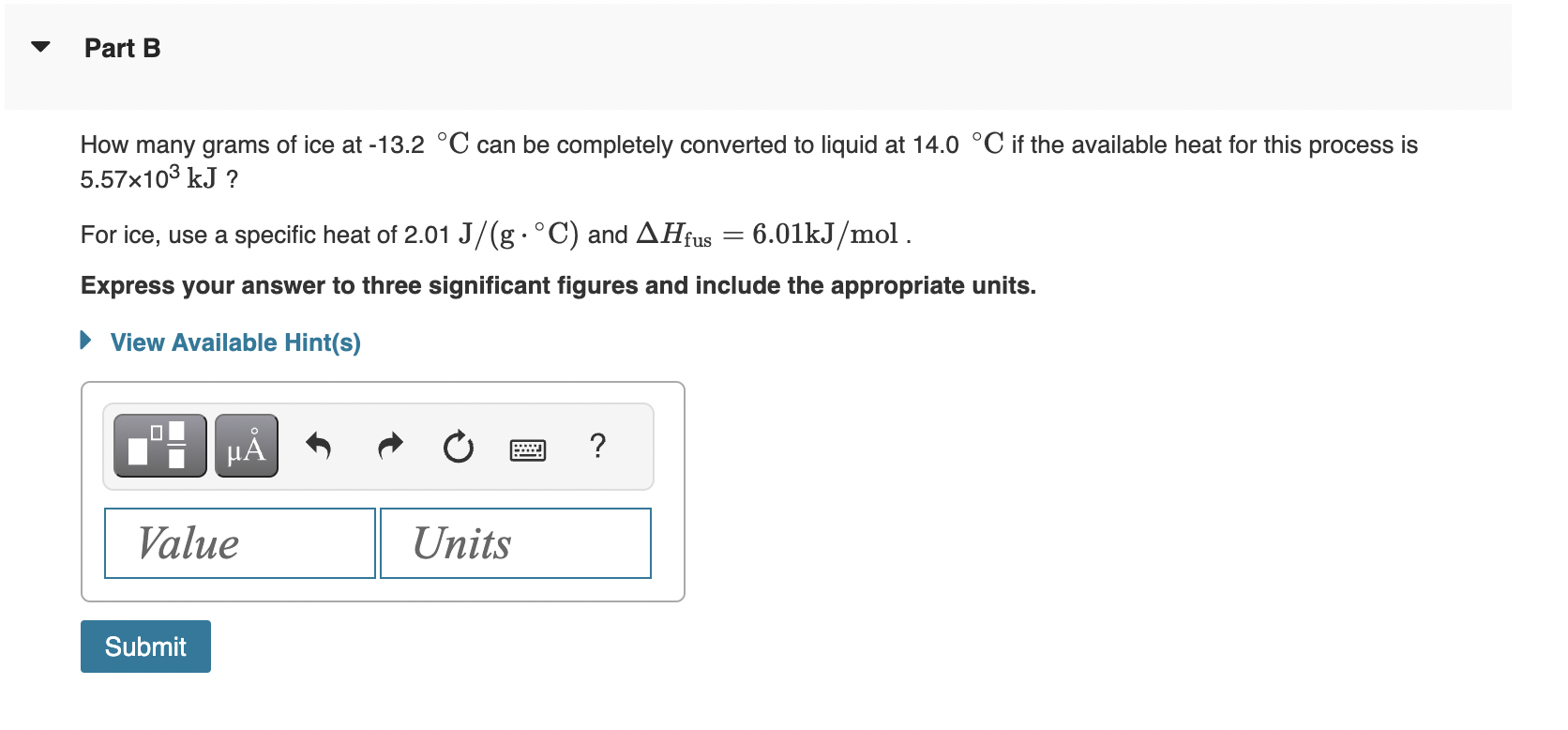
Calculate the enthalpy change, H, for the process in which 21.6g of water is converted from liquid at 19.6C to vapor at 25.0C. For water, Hvap=44.0kJ/mol at 25.0C and Cs=4.18J/(gC) for H2O(l). Express your answer to three significant figures and include the appropriate units. How many grams of ice at 13.2C can be completely converted to liquid at 14.0C if the available heat for this process is 5.57103kJ ? For ice, use a specific heat of 2.01J/(gC) and Hfus=6.01kJ/mol. Express your answer to three significant figures and include the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


