Question: Q.30 Consider the reaction H2 + C2H4 C2H6 The molecular diameters of H2 and C,H4 are 1.8 and 3.6 respectively. The pre-exponential factor in
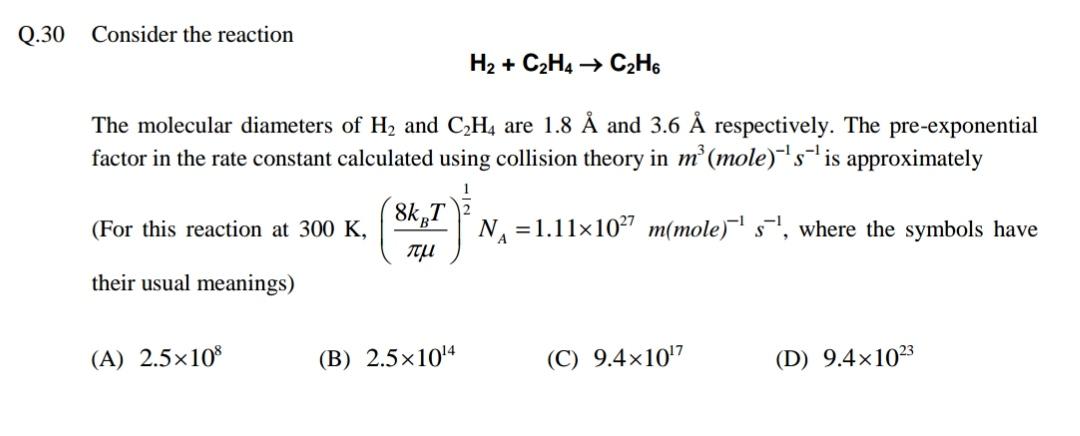
Q.30 Consider the reaction H2 + C2H4 C2H6 The molecular diameters of H2 and C,H4 are 1.8 and 3.6 respectively. The pre-exponential factor in the rate constant calculated using collision theory in m (mole)sis approximately 8kT 7 (For this reaction at 300 K, N =1.11x107 m(mole) s', where the symbols have their usual meanings) (A) 2.5x10 (B) 2.5x1014 (C) 9.410'7 (D) 9.41023
Step by Step Solution
3.47 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


