Question: Calculations for Unknown Solute: Work backwards using the information in this table to identify the solute from the Table 2 1. Name of unknown solute
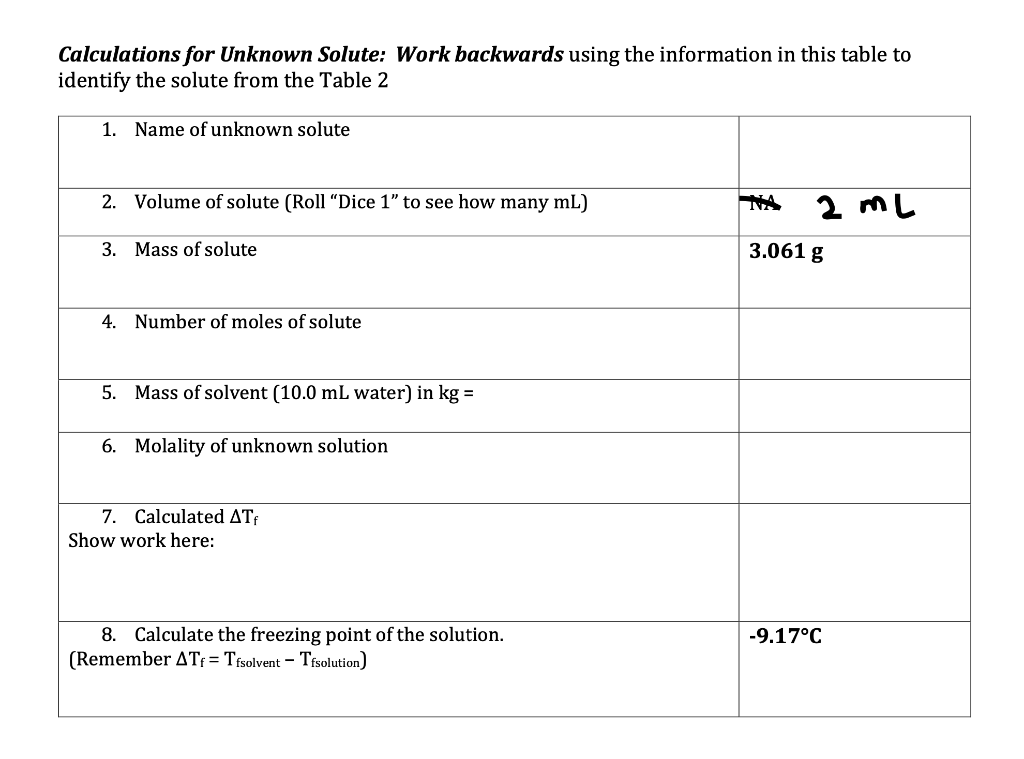
Calculations for Unknown Solute: Work backwards using the information in this table to identify the solute from the Table 2 1. Name of unknown solute 2. Volume of solute (Roll Dice 1" to see how many mL) 2 mL 3. Mass of solute 3.061 g 4. Number of moles of solute 5. Mass of solvent (10.0 mL water) in kg = 6. Molality of unknown solution 7. Calculated ATF Show work here: -9.17C 8. Calculate the freezing point of the solution. (Remember AT = Tfsolvent - Tfsolution) Calculations for Unknown Solute: Work backwards using the information in this table to identify the solute from the Table 2 1. Name of unknown solute 2. Volume of solute (Roll Dice 1" to see how many mL) 2 mL 3. Mass of solute 3.061 g 4. Number of moles of solute 5. Mass of solvent (10.0 mL water) in kg = 6. Molality of unknown solution 7. Calculated ATF Show work here: -9.17C 8. Calculate the freezing point of the solution. (Remember AT = Tfsolvent - Tfsolution)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


