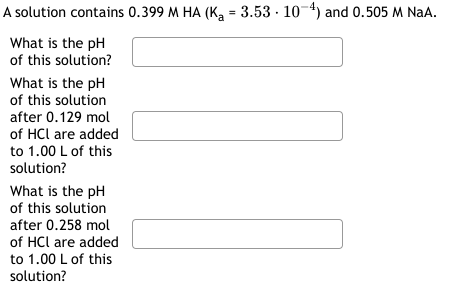
Question: Can anyone help me with this question? A solution contains 0.399MHA(Ka=3.53104) and 0.505MNaA. What is the pH of this solution? What is the pH of
 Can anyone help me with this question?
Can anyone help me with this question?
A solution contains 0.399MHA(Ka=3.53104) and 0.505MNaA. What is the pH of this solution? What is the pH of this solution after 0.129mol of HCl are added to 1.00L of this solution? What is the pH of this solution after 0.258mol of HCl are added to 1.00L of this solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


