Question: Hydrogen gas reduces NO to N in the following reaction: 2 H(g) + 2 NO(g) 2 HO(g) + N(g) The initial reaction rates of
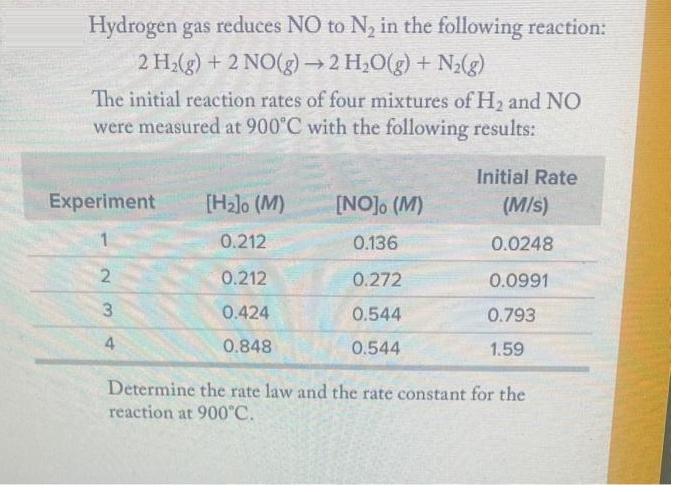
Hydrogen gas reduces NO to N in the following reaction: 2 H(g) + 2 NO(g) 2 HO(g) + N(g) The initial reaction rates of four mixtures of H and NO were measured at 900C with the following results: Experiment 2 3 4 [H]o (M) 0.212 0.212 0.424 0.848 [NO], (M) 0.136 0.272 0.544 0.544 Initial Rate (M/s) 0.0248 0.0991 0.793 1.59 Determine the rate law and the rate constant for the reaction at 900C.
Step by Step Solution
3.59 Rating (167 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


