Question: Can someone do this plz ? 4 Consider the following reactions of four metals - D, Z, Q and X. Q(3) + X(NO3)2(aq) Q(NO3)2(aq) +
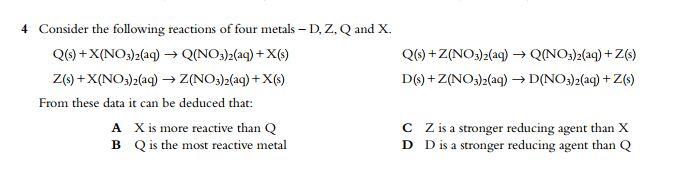 Can someone do this plz ?
Can someone do this plz ?
4 Consider the following reactions of four metals - D, Z, Q and X. Q(3) + X(NO3)2(aq) Q(NO3)2(aq) + X() Z(9)+X(NO3)2(aq) Z(NO3)2(aq) + X(3) From these data it can be deduced that: A X is more reactive than Q B Q is the most reactive metal Q(s) +Z(NO3)2(aq) Q(NO3)2(aq) +Z() D(3) + Z(NO3)2(aq) D(NO3)2(aq) +Z(3) CZ is a stronger reducing agent than X D D is a stronger reducing agent than
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


