Question: can someone help me with question 2 part b. ng Imol 0.0399 24.3059 Table 1: Reactants and Products Mass moles Name of Compound MPt or
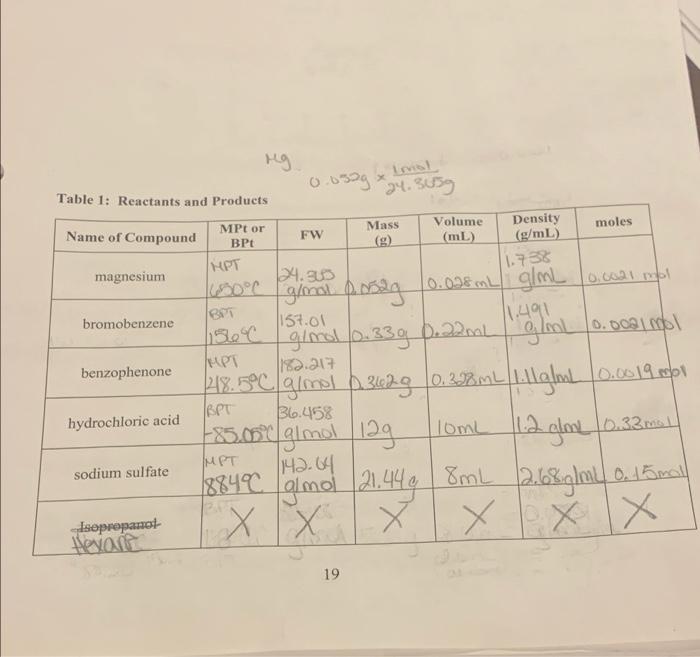

ng Imol 0.0399 24.3059 Table 1: Reactants and Products Mass moles Name of Compound MPt or BPt Density (g/mL) Volume (mL) FW THPT magnesium 24.900 BPT bromobenzene 1157.01 140.00am MPT benzophenone 1182.217 1.738 ginal posag 10.028 ml g/mL 10.0021 mol mL ginal (0.33g D22ml 48.5C glmal 63628 0.272mL Liglo 0.0019 obol -85.000 gimol 12g 11.2 glov (0.33ml 1142.64 188400 gimol (21.449 | 8mL (2.68 g/mL o. 15 mall XX x X X X IBPT 36.458 hydrochloric acid 10mL IMPT sodium sulfate Lopropanol Havalt 19 1. Draw the results of your TIC data on the TLC plate below. 0 (1 - bromobenzene, 2 - benzophenone, 3 - crude mixture, 4 recrystallized product) 2. (a) Amount of recrystallized triphenolmethanol produced in grams, O.JE, (b) Calculate your percent yield. Ax 100% T 0.124g x 100% 3. (a) Melting Point (MPL) of your triphenylmethanol 15575527c. (b) Literature MPt. of triphenylmethanol 10-163 C (Please cite your source below), Santa Cruz Biolechnology https://www.sobot. complete pronylonsthanol-76-846 I couldn't find 1574 online, 21
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


