Question: Can someone please answer question 25 and 28 Use the information provided to determine Hrxn for the following reaction: Hf(kJ/mol)+CO2(g)Fe2O3(s)+CO(g)2Fe3O4(s)Fe2O3(s)824Fe3O4(s)1118CO(g)111CO2(g)394 A) 577kJ B) 47kJ C)
Can someone please answer question 25 and 28

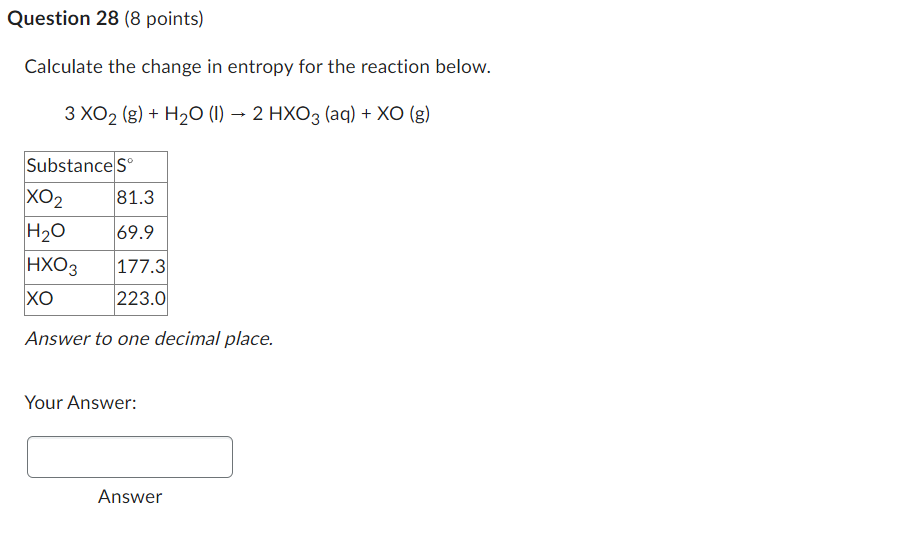
Use the information provided to determine Hrxn for the following reaction: Hf(kJ/mol)+CO2(g)Fe2O3(s)+CO(g)2Fe3O4(s)Fe2O3(s)824Fe3O4(s)1118CO(g)111CO2(g)394 A) 577kJ B) 47kJ C) +277kJ D) 111kJ E) +144kJ Calculate the change in entropy for the reaction below. 3XO2(g)+H2O(l)2HXO3(aq)+XO(g) Answer to one decimal place. Your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


