Question: Can someone please help me solve this! I am having a hard time and I've been trying all day :( Empirical Formula calculation: Empirical Formula:
Can someone please help me solve this! I am having a hard time and I've been trying all day :(
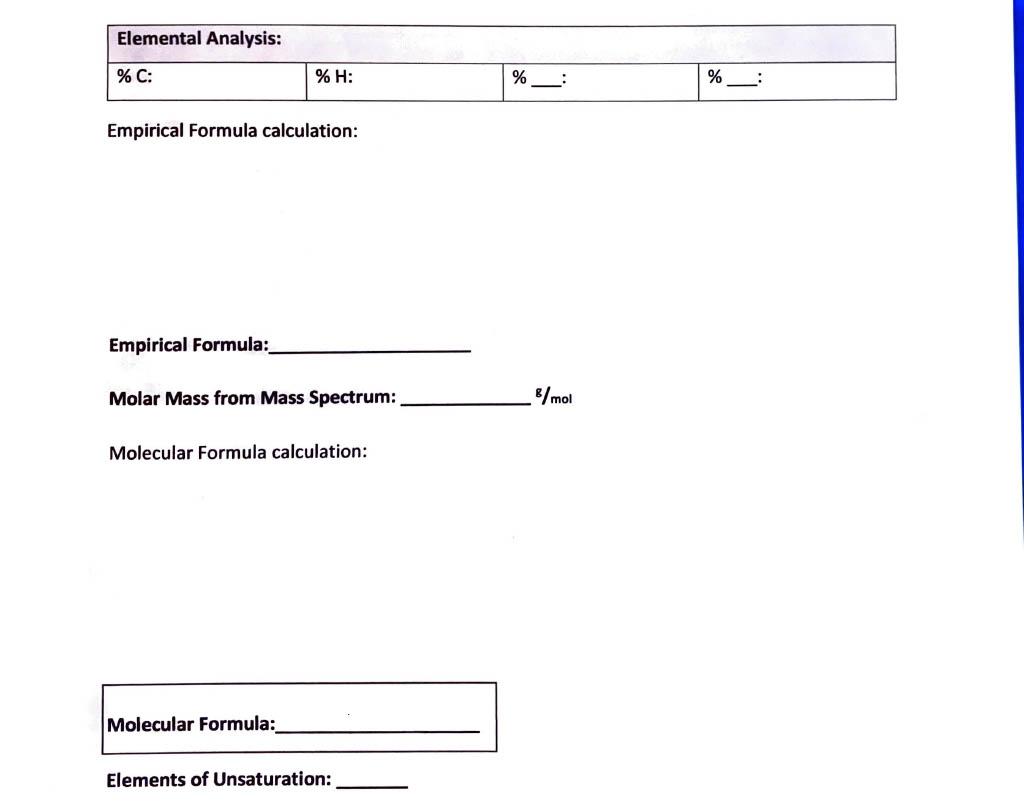
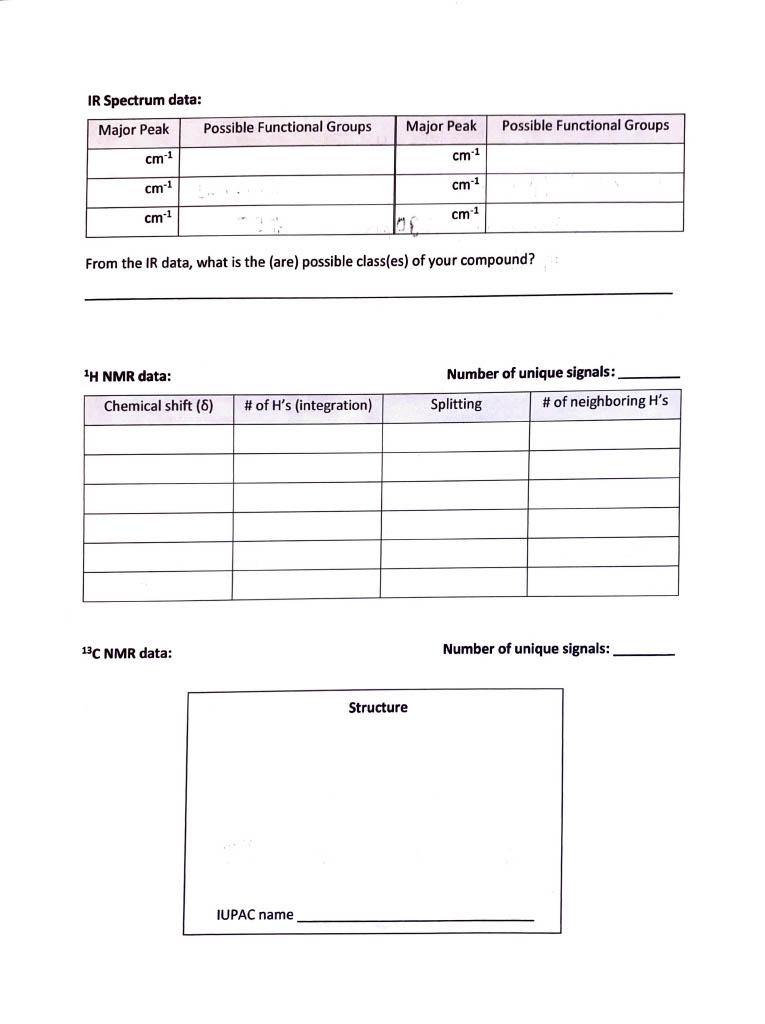
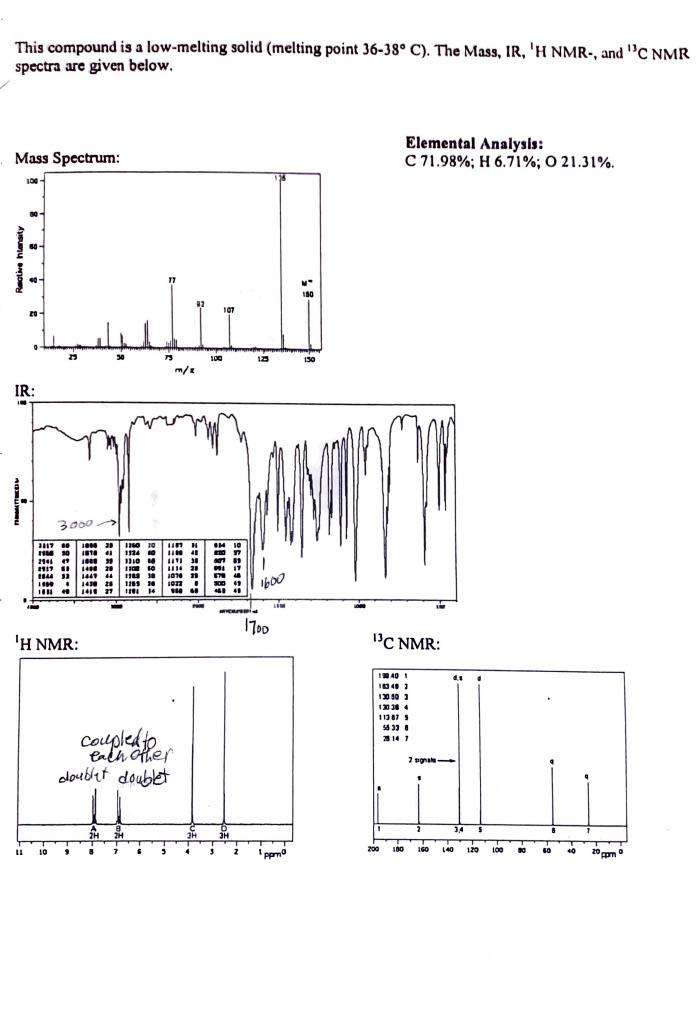
Empirical Formula calculation: Empirical Formula: Molar Mass from Mass Spectrum: g/mol Molecular Formula calculation: Molecular Formula: Elements of Unsaturation: IR Spectrum data: From the IR data, what is the (are) possible class(es) of your compound? 1H NMR data: Number of unique signals: 13 C NMR data: Number of unique signals: This compound is a low-melting solid (melting point 3638C ). The Mass, IR, ' HNMR, and 13CNMR spectra are given below. Elemental Analysis: C 71.98\%; H 6.71\%; O 21.31\%. 'H NMR. 13 C NMR
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


