Question: can someone please help with c-g please its supposed to be done on excell but i am not understanding it. (12 pts) Lola is conducting
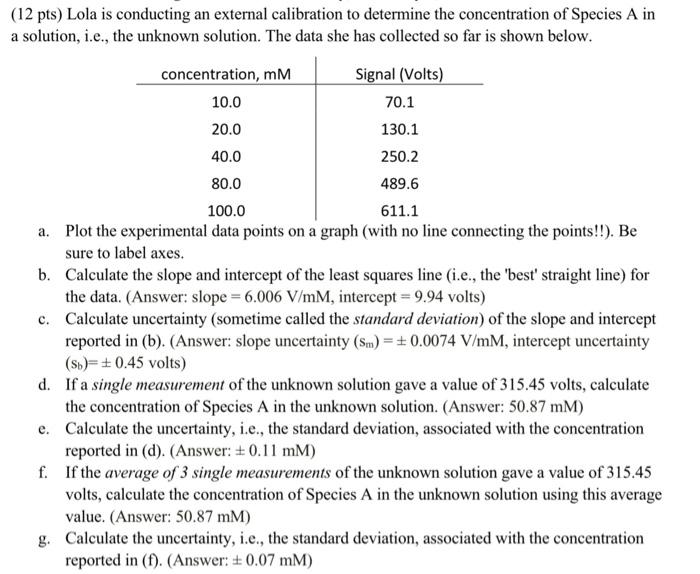
(12 pts) Lola is conducting an external calibration to determine the concentration of Species A in a solution, i.e., the unknown solution. The data she has collected so far is shown below. concentration, mM Signal (Volts) 10.0 70.1 20.0 130.1 40.0 250.2 80.0 489.6 100.0 611.1 a. Plot the experimental data points on a graph (with no line connecting the points!!). Be sure to label axes. b. Calculate the slope and intercept of the least squares line (i.e., the 'best' straight line) for the data. (Answer: slope = 6.006 V/mM, intercept = 9.94 volts) c. Calculate uncertainty (sometime called the standard deviation) of the slope and intercept reported in (b). (Answer: slope uncertainty (Sm) = +0.0074 V/mM, intercept uncertainty (Sb=+0.45 volts) d. If a single measurement of the unknown solution gave a value of 315.45 volts, calculate the concentration of Species A in the unknown solution. (Answer: 50.87 mm) e. Calculate the uncertainty, i.e., the standard deviation, associated with the concentration reported in (d). (Answer: +0.11 mm) f. If the average of 3 single measurements of the unknown solution gave a value of 315.45 volts, calculate the concentration of Species A in the unknown solution using this average value. (Answer: 50.87 mm) g. Calculate the uncertainty, i.e., the standard deviation, associated with the concentration reported in (f). (Answer: +0.07 mm)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


