Question: Can someone solve the bottom one Consider the first-order reaction described by the equation H H.C ECH, HC H H Cyclopropane Propene At a certain
Can someone solve the bottom one 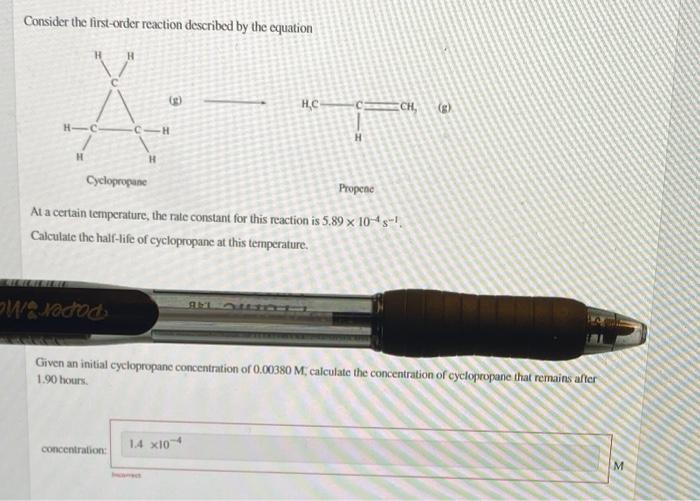

Consider the first-order reaction described by the equation H H.C ECH, HC H H Cyclopropane Propene At a certain temperature, the rate constant for this reaction is 5.89 x 10-45-1 Calculate the half-life of cyclopropane at this temperature. Nadod Given an initial cyclopropane concentration of 0.00380 M, calculate the concentration of cyclopropane that remains aller 1.90 hours concentration: 1.4 X10- M
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


