Question: can u please show the steps and highlight the answer Consider the reaction: A2(g) + 6 B(8) E 2 AB3 (8) A student attempted to
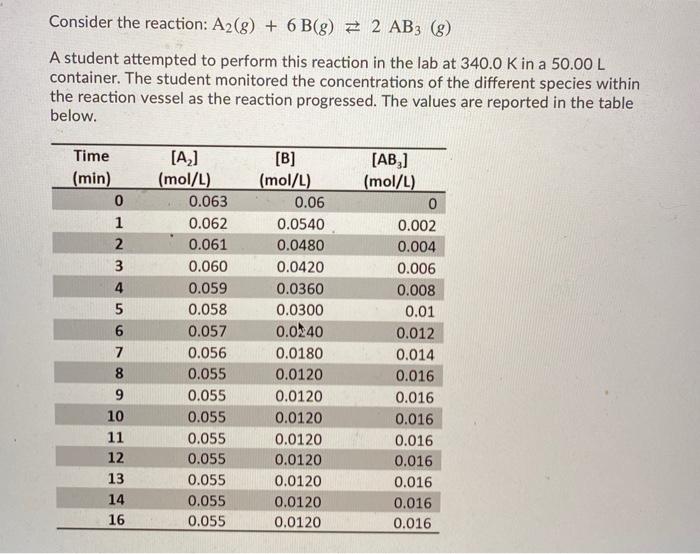

Consider the reaction: A2(g) + 6 B(8) E 2 AB3 (8) A student attempted to perform this reaction in the lab at 340.0 K in a 50.00 L container. The student monitored the concentrations of the different species within the reaction vessel as the reaction progressed. The values are reported in the table below. Time (min) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 16 [A] (mol/L) 0.063 0.062 0.061 0.060 0.059 0.058 0.057 0.056 0.055 0.055 0.055 0.055 0.055 0.055 0.055 0.055 [B] (mol/L) 0.06 0.0540 0.0480 0.0420 0.0360 0.0300 0.0240 0.0180 0.0120 0.0120 0.0120 0.0120 0.0120 0.0120 0.0120 0.0120 [AB] (mol/L) 0 0.002 0.004 0.006 0.008 0.01 0.012 0.014 0.016 0.016 0.016 0.016 0.016 0.016 0.016 0.016 Determine the partial pressure (in kPa) of the B reactant 2.0 min after the start of the reaction. Report your answer with 3 significant figures; do not use the scientific notation and do not include units in your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


