Question: can you explain step by step how you got the correct answer Nitrogen monoxide reacts with bromine at elevated temperatures according to the equation 2NO(g)+Br2(g)2NOBr(g)
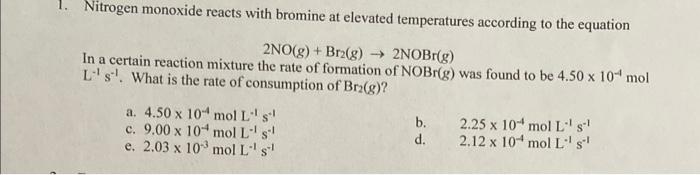
Nitrogen monoxide reacts with bromine at elevated temperatures according to the equation 2NO(g)+Br2(g)2NOBr(g) In a certain reaction mixture the rate of formation of NOBr(g) was found to be 4.50104mol L1s1. What is the rate of consumption of Br2(g) ? a. 4.50104molL1s1 c. 9.00104molL1s1 e. 2.03103molL1s1 b. 2.25104molL1s1 d. 2.12104molL1s1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


