Question: can you explain this aqueous solution, hypobromite ion, BrO, reacts to produce bromate ion, BrO3, and bromide on, Br, according to the following chemical equation.
can you explain this 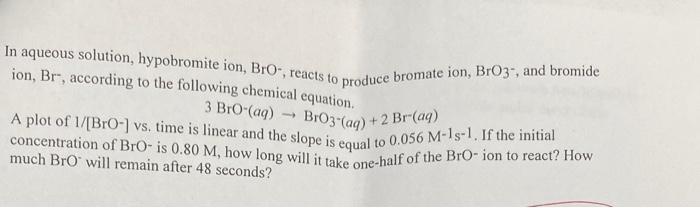
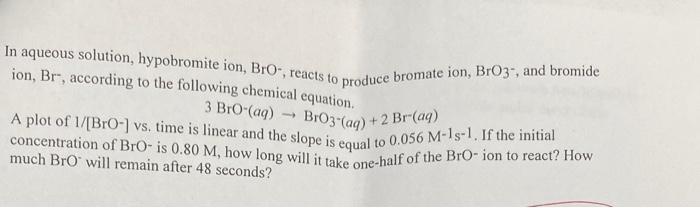
aqueous solution, hypobromite ion, BrO, reacts to produce bromate ion, BrO3, and bromide on, Br, according to the following chemical equation. A plot of 1/[BrO-] vs, time is linear and the slope is equal to 0.056BrO(aq)BrO31(aq)+2Br(aq). If the initial oncentration of BrOis 0.80M, how long will it take one-half of the BrO - ion to react? How nuch BrOwill remain after 48 seconds
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


