Question: Can you please help me figure out this problem. 13. (8 points) A monatomic ideal gas is taken through the cycle shown on the pressure
Can you please help me figure out this problem.
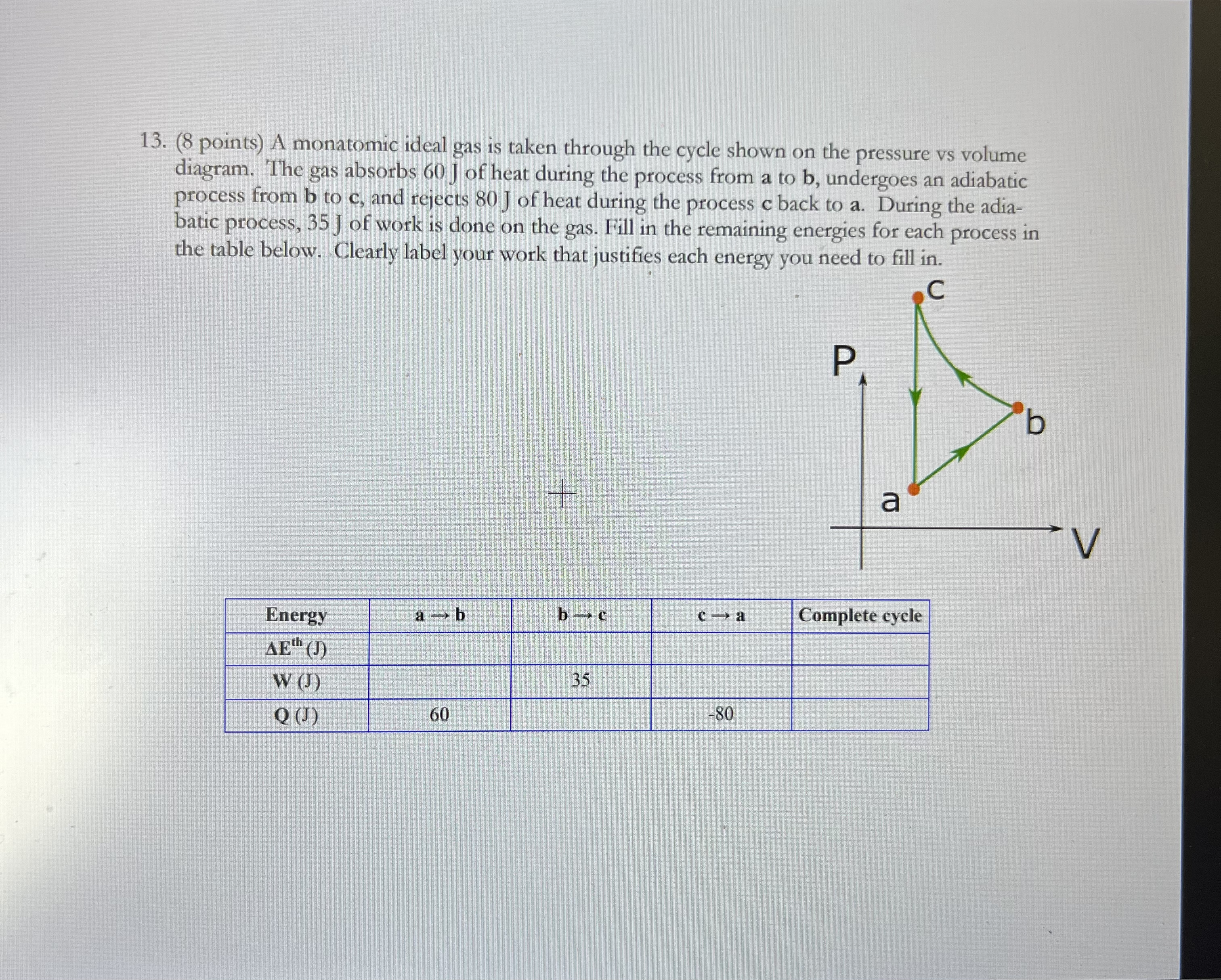
13. (8 points) A monatomic ideal gas is taken through the cycle shown on the pressure vs volume diagram. The gas absorbs 60 J of heat during the process from a to b, undergoes an adiabatic process from b to c, and rejects 80 J of heat during the process c back to a. During the adia- batic process, 35 J of work is done on the gas. Fill in the remaining energies for each process in the table below. Clearly label your work that justifies each energy you need to fill in. C P a V Energy 1-b b - c c - a Complete cycle AE" (J) W (J 35 Q() 60 -80
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


