Question: Can you please help me with this question? Thank you. Rate Law from Concentration versus Time Data NO2(g)+CO(g)NO(g)+CO2(g) The rate of the above reaction depends
Can you please help me with this question? Thank you. 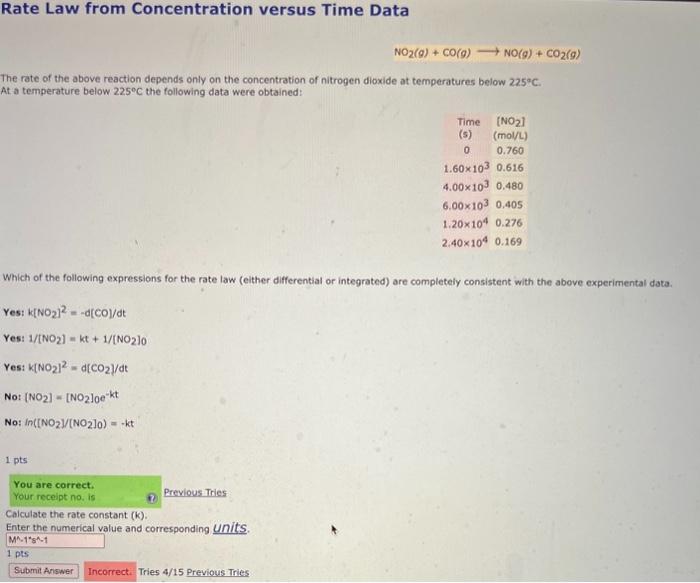

Rate Law from Concentration versus Time Data NO2(g)+CO(g)NO(g)+CO2(g) The rate of the above reaction depends only on the concentration of nitrogen dioxide at temperatures below 225C. At a temperature below 225C the following data were obtained: Which of the following expressions for the rate law (either differential or integrated) are completely consistent with the above experimental data. Yes:k[NO2]2=d[CO]/dtYes:1/[NO2]=kt+1/[NO2]0Yes:k[NO2]2=d[CO2]/dtNo:[NO2]=[NO2]0ektNo:In[[NO2]/(NO2]0)=kt 1 pts Calculate the rate constant (k). Enter the numerical value and corresponding units. 1pts Tries 4/15 Previous Tries
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


