Question: can you please solve it with drawing thanks - Consider a binary system of benzene (A) and toluene (B). - If 100 moles of a

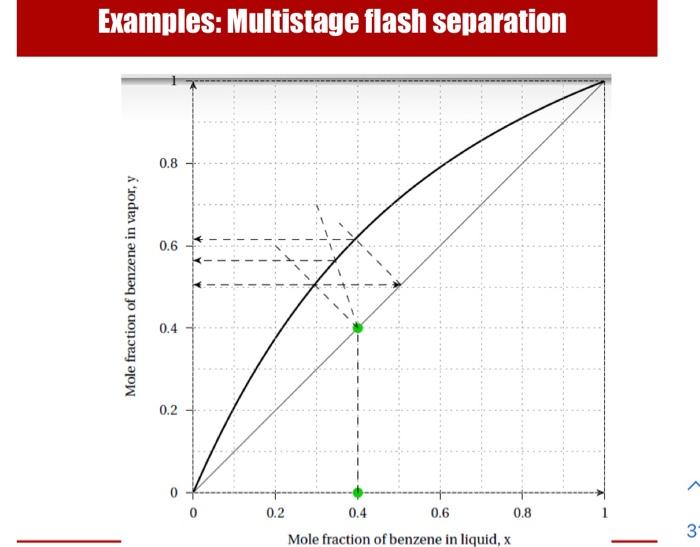
- Consider a binary system of benzene (A) and toluene (B). - If 100 moles of a feed mixture containing 40%A is vaporized to produce 25 moles of vapor, what will be the vapor composition? - If instead of vaporizing 25 moles, in the first step 50 moles are vaporized and the vapor is condensed. In the second step, if 25 moles from the condensate is vaporized, what will be the composition of the resulting vapor? Examples: Multistage flash separation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


