Question: Can you pls answer this and show all your work. I need to confirm my work. thanks! Table 1: The Colour of Vanadium or Vanadate
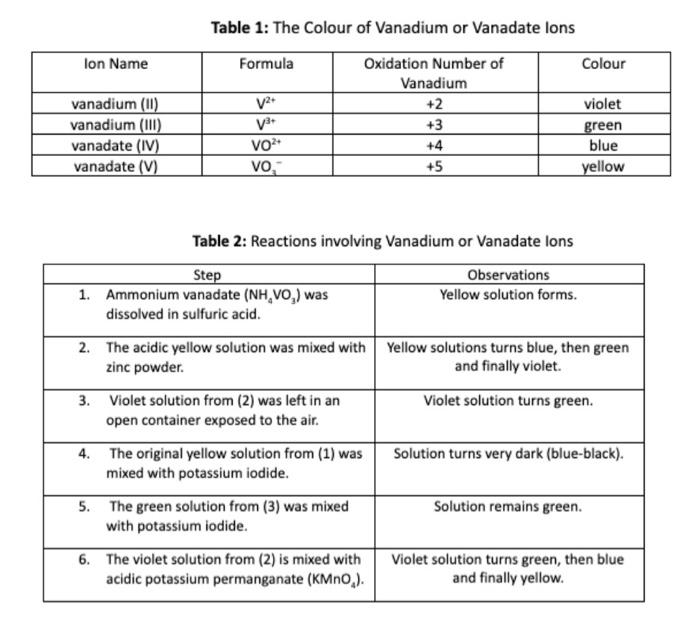
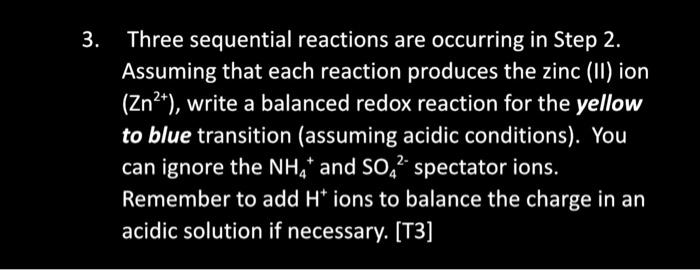
Table 1: The Colour of Vanadium or Vanadate Ions Table 2: Reactions involving Vanadium or Vanadate lons 3. Three sequential reactions are occurring in Step 2. Assuming that each reaction produces the zinc (II) ion (Zn2+), write a balanced redox reaction for the yellow to blue transition (assuming acidic conditions). You can ignore the NH4+and SO42 spectator ions. Remember to add H+ions to balance the charge in an acidic solution if necessary. [T3]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


