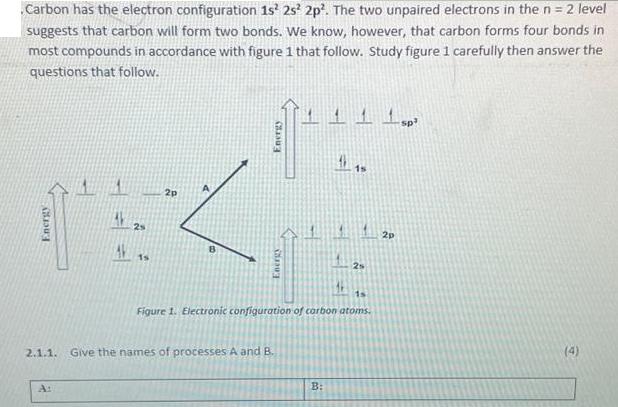
Carbon has the electron configuration 1s 2s2p. The two unpaired electrons in the n = 2...
Fantastic news! We've Found the answer you've been seeking!
Question:
Transcribed Image Text:
Carbon has the electron configuration 1s 2s²2p². The two unpaired electrons in the n = 2 level suggests that carbon will form two bonds. We know, however, that carbon forms four bonds in most compounds in accordance with figure 1 that follow. Study figure 1 carefully then answer the questions that follow. Energy 2s 15 2p Energy Energy 2.1.1. Give the names of processes A and B. 115 1s Figure 1. Electronic configuration of carbon atoms. B: 2 1 sp³ 2p (4) Carbon has the electron configuration 1s 2s²2p². The two unpaired electrons in the n = 2 level suggests that carbon will form two bonds. We know, however, that carbon forms four bonds in most compounds in accordance with figure 1 that follow. Study figure 1 carefully then answer the questions that follow. Energy 2s 15 2p Energy Energy 2.1.1. Give the names of processes A and B. 115 1s Figure 1. Electronic configuration of carbon atoms. B: 2 1 sp³ 2p (4)
Expert Answer:
Answer rating: 100% (QA)
4 Hybridization and Promotion of electrons respectively 212 What are the numbers of electrons involv... View the full answer

Related Book For 

Smith and Roberson Business Law
ISBN: 978-0538473637
15th Edition
Authors: Richard A. Mann, Barry S. Roberts
Posted Date:
Students also viewed these accounting questions
-
A certain oxygen atom has the electron configuration 1s22s22px22py2. How many unpaired electrons are present? Is this an excited state for oxygen? In going from this state to the ground state, would...
-
In the book Advanced Managerial Accounting, Robert P. Magee discusses monitoring cost variances. A cost variance is the difference between a budgeted cost and an actual cost. Magee describes the...
-
The owner of Atlantic City Confectionary Is considering the purchase of a new semiautomatic candy machine. The machine will cost $24,000 and last 10 years. The machine is expected to have no salvage...
-
Your client has offered a 5-year, $1,000 par value bond with a 10 percent coupon. Interest on this bond is paid quarterly. 1) If your client is to earn a nominal rate of return of 12 percent,...
-
A car dealership expects to sell 6 cars per day, with standard deviation of 2.1 cars per day. During a 100-day period, estimate the probability that they sell strictly more than 625 cars.
-
First, explain to those in the meeting the concept of social responsibility in terms of how it applies to New Balances consideration to expand sales into these new product lines. Using this concept,...
-
Light through the water Some college students collected data on the intensity of light at various depths in a lake. Here are their data: Depth (m) Light intensity (lumens) 5 168.00 6 120.42 7 86.31 8...
-
Results from First Corporations most recent year of operations is presented in the following table. Operating income.......................................... $ 7,560 Total...
-
Blue's Tires can issue perpetual preferred stock at a price of $67 a share. The stock would pay a constant annual dividend of $6.00 a share. What is the company's cost of preferred stock?
-
A conducting sphere of radius a is half-embedded in a liquid dielectric medium of permittivity s, as in figure. The region above the liquid is a gas of permittivity ?2. If the total free charge on...
-
11) pls help asap! thanks Current Attempt in Progress Oriole Corporation's comparative balance sheets are presented below. Additional information: 1. Net income was $22,600. Dividends declared and...
-
In the introduction to "The Five Sexes," Anne Fausto-Sterling writes that she had to "invent conventions - s/he and his/her - to denote someone who is clearly neither male nor female or who is...
-
Select a product described as one of the "Biggest Product Flops" of 2019 that you will bring back to the market. To, you will need to engage in some research to understand why the product failed to...
-
Breaking the Bank Case Questions (video found at: http://www.pbs.org/wgbh/pages/frontline/breakingthebank/view/?utm_campaign=viewpage &utm_medium=grid&utm_source=grid) 1) To what extent were the...
-
Please answer in full and write legibly. Suppose Alice has taken 7 classes college, and her current GPA is 3.48 (assume for simplicity that all courses carry the same number of credits). Answer the...
-
F. Explain how to overcome two potential biases (e.g., prejudice, discrimination) using culturally competent strategies that will help improve stakeholder communication. G. Explain how to mitigate...
-
IBM expects to pay a dividend of $3 next year and expects these dividends to grow at 8% a year. The price of IBM is $80 per share. Your estimate of the market risk premium is 9%. The risk-free rate...
-
The maximum pressure that can be developed for a certain fluid power cylinder is 15.0 MPa. Compute the required diameter for the piston if the cylinder must exert a force of 30 kN.
-
In 1963 the Saturday Evening Post featured an article entitled The Story of a College Football Fix, characterized in the subtitle as A Shocking Report of How Wally Butts and Bear Bryant Rigged a Game...
-
This is a stocklist case arising under 220(b) of our [Delaware] General Corporation Law. The issue is whether a shareholder states a proper purpose for inspection under our statute in seeking to...
-
While driving his car in Virginia, Carpe Diem, a resident of North Carolina, struck Butt, a resident of Alaska. As a result of the accident, Butt suffered more than $60,000 in medical expenses. Butt...
-
Espresso Express operates a number of espresso coffee stands in busy suburban malls. The fixed weekly expense of a coffee stand is $1,200 and the variable cost per cup of coffee served is $0.22....
-
variable costs? Give several examples of activity bases.
-
What is meant by an activity base when dealing with

Study smarter with the SolutionInn App


