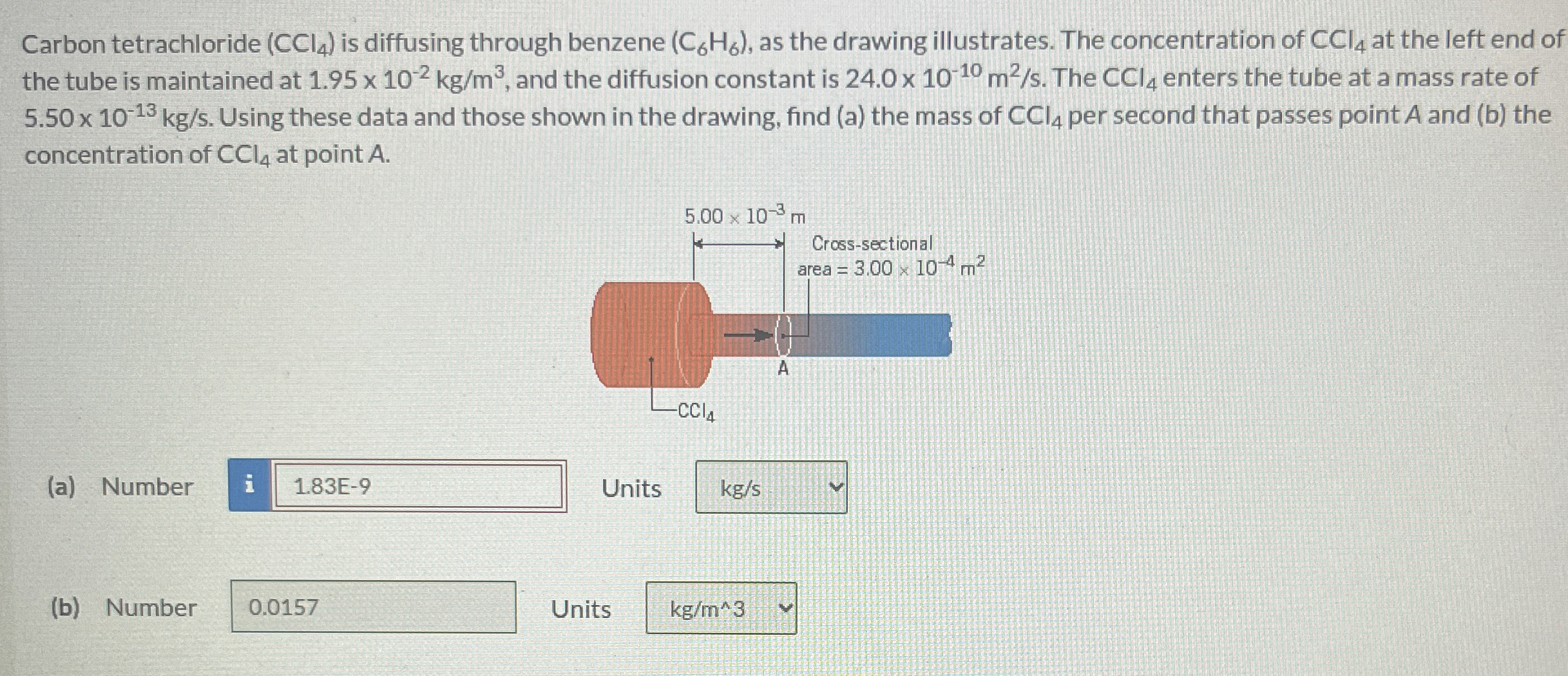
Question: Carbon tetrachloride ( C C l 4 ) is diffusing through benzene ( C 6 H 6 ) , as the drawing illustrates. The concentration
Carbon tetrachloride is diffusing through benzene as the drawing illustrates. The concentration of at the left end of the tube is maintained at and the diffusion constant is The enters the tube at a mass rate of Using these data and those shown in the drawing, find a the mass of per second that passes point A and b the concentration of at point
a Number Units
b Number Units
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


