Question: CE2REE 1. SECTION A - Answer ALL questions from this section The elementary reaction A+B - 2P takes place in the gas phase at 200
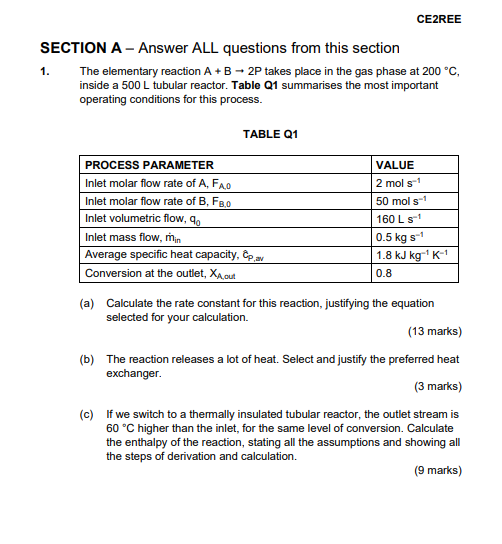
CE2REE 1. SECTION A - Answer ALL questions from this section The elementary reaction A+B - 2P takes place in the gas phase at 200 C, inside a 500 L tubular reactor. Table Q1 summarises the most important operating conditions for this process. TABLE Q1 PROCESS PARAMETER VALUE Inlet molar flow rate of A, FAO 2 mol s1 Inlet molar flow rate of B, F8,0 50 mols-1 Inlet volumetric flow,90 160 L S- Inlet mass flow, min 0.5 kg st Average specific heat capacity, Cp av 1.8 kJ kg 'K- Conversion at the outlet, XA cut 0.8 (a) Calculate the rate constant for this reaction, justifying the equation selected for your calculation. (13 marks) (b) The reaction releases a lot of heat. Select and justify the preferred heat exchanger. (3 marks) (c) If we switch to a thermally insulated tubular reactor, the outlet stream is 60 C higher than the inlet, for the same level of conversion. Calculate the enthalpy of the reaction, stating all the assumptions and showing all the steps of derivation and calculation. (9 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


