Question: Chapter 13 Res! C Question 1 Tor This question has multiple parts. Work all the parts to get the most points. Question for Duration 3
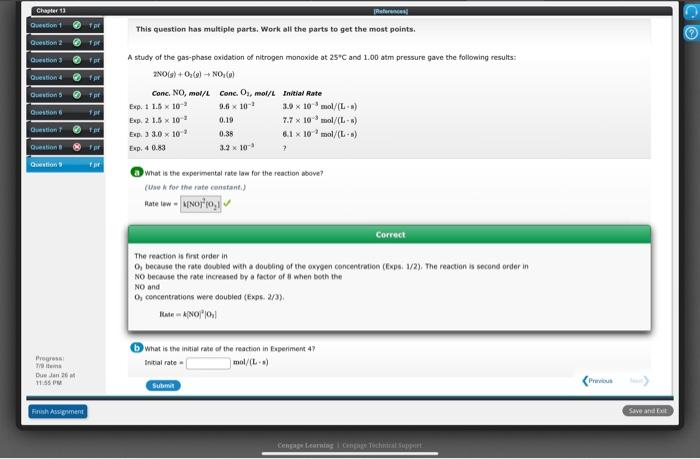
Chapter 13 Res! C Question 1 Tor This question has multiple parts. Work all the parts to get the most points. Question for Duration 3 Question 4 pr Questions pr A study of the gas-phase oxidation of nitrogen monoxide at 25C and 1.00 atm pressure gave the following results: 2NOG) +0,6) NO:6) Conc. NO, mol/l Conc. O, mol/L Initial Rate Exp. 1 1.5 x 10- 9.6 x 10-1 3.9 x 10 mol/(L) Exp. 2 1.5 x 10" 0.19 7.7 * 10 mol/L) Exp. 33.0 x 10" 0.38 6.1 x 10 mol/L) Exp. 40.80 3.2 x 10- Questions 19 stion Question or Gestion What is the experimental rate law for the reaction above? (the for the rate constant) Hate wwwporio Correct The reaction is first order in O, because the rate doubled with a doubling of the oxygen concentration (Exp. 1/2). The reaction is second order in NO because the rate increased by a factor of when both the NO and O concentrations were doubled (Expe. 2/3) Rate - Ano"10:11 What is the initial rate of the reaction in Experiment 47 Initial rate mol/L) Press Trom De JM 11:55 PM P Sub Fish Assigment Save and bet Cengage learning Contoh Support
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


