Question: Chart is below: 6. Locate the numbers in Model 1 that repreient the ioniration entrgy. The ionization energy it the amouns of energy needed to

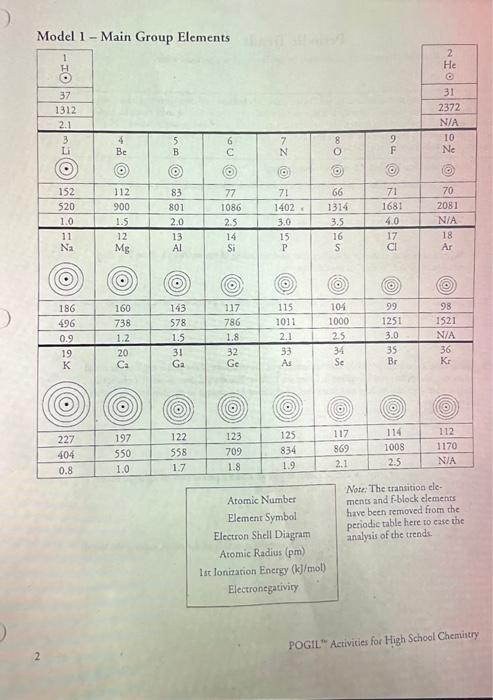
6. Locate the numbers in Model 1 that repreient the ioniration entrgy. The ionization energy it the amouns of energy needed to remove an electron from an atom. a. Using your knowledge of Coulombic atraction, explain why ioniration-temening an electron from an atom-taker energy: b. Which cakes more energy, removing an clecuron from an atom where the nadeus has a tighis hold on its electrons, or a weak hold on its clectrons? Explain. 7. In general, what is the trend in ionization energy as you go down a group? Support your answer using examples from three groups. 8. Using your knowledge of Coulombic artaction and the structure of the atom, explain the trend in ionization energy that you identified in Quertion 7. 9. In general, what is the trend in ionizarion energy as you go across a period? Support your answer using examples from two petiods. 10. Using your knowledge of Coulombic attraction and the structure of the atom, explain the trend in ionization energy that you identified in Question 9. 11. Aroms with loosely held electrons are urually classified as metals. They will exhibir high conductivity, ductility, and malleability because of their atomic structure. Would you expect metals to have high ionization energies or low ionization energies? Explain your answer in one to two complete sentences. POGIL" Activities for High School Chemintry
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


