Question: Chat GPT answers will be reported. Al software will be reported. Do not copy answers from elsewhere, work manually. 4. A new monoprotic pH-sensitive dye
Chat GPT answers will be reported. Al software will be reported. Do not copy answers from elsewhere, work manually.
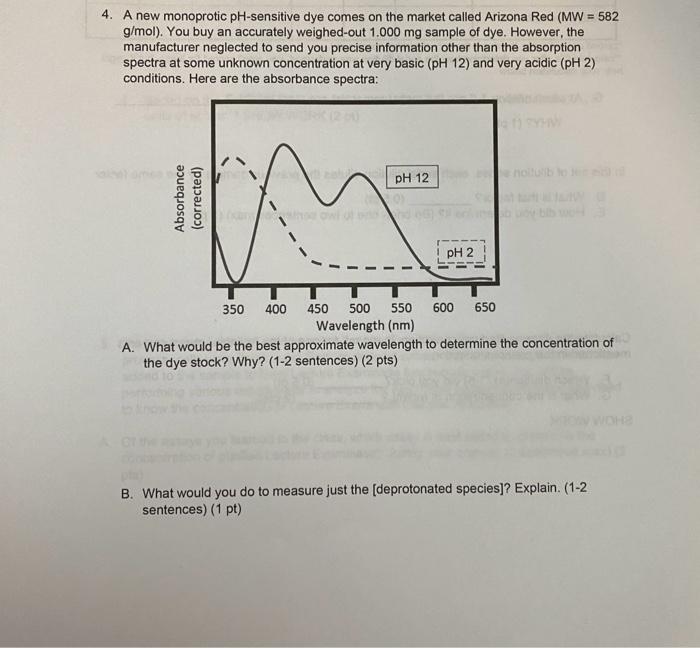
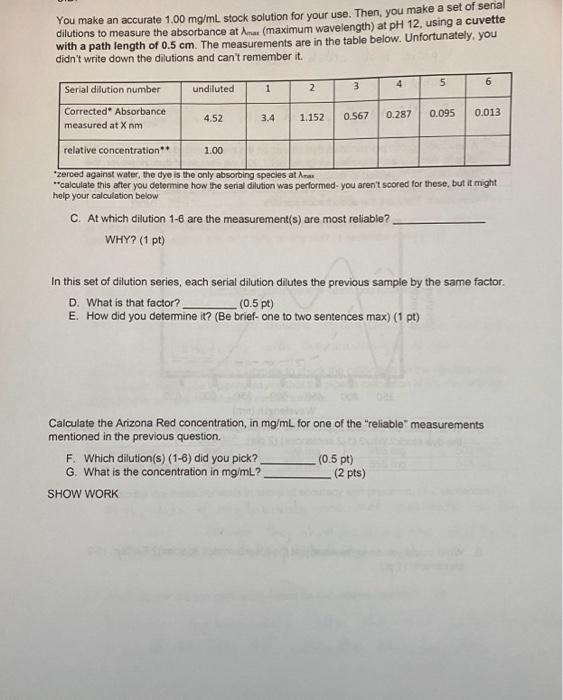

4. A new monoprotic pH-sensitive dye comes on the market called Arizona Red ( MW=582 g/mol). You buy an accurately weighed-out 1.000mg sample of dye. However, the manufacturer neglected to send you precise information other than the absorption spectra at some unknown concentration at very basic (pH12) and very acidic (pH2) conditions. Here are the absorbance spectra: A. What would be the best approximate wavelength to determine the concentration of the dye stock? Why? (1-2 sentences) (2 pts) B. What would you do to measure just the [deprotonated species]? Explain. (1-2 sentences) (1 pt) You make an accurate 1.00mg/mL stock solution for your use. Then, you make a set of serial with a path length of 0.5cm. The measurements are in the table below. Unfortunately, you didn't write down the dilutions and can't remember it. "zeroed against water, the dye is the only absorting specles at rax "calculate this atter you detormine how the serial dilution was performed- you aren't soored tor these, but it might help your calculation below C. At which dilution 1-6 are the measurement(s) are most reliable? WHY? (1 pt) In this set of dilution series, each serial dilution dilutes the previous sample by the same factor. D. What is that factor? (0.5pt) E. How did you determine it? (Be brief-one to two sentences max) (1 pt) Calculate the Arizona Red concentration, in mg/mL for one of the "reliable" measurements mentioned in the previous question. F. Which dilution(s) (1-6) did you pick? (0.5pt) G. What is the concentration in mg/mL ? (2 pts) SHOW WORK H. What is the absorption coefficient at max in units of (mg/ml)1cm1 ? SHOW WORK (3 pts) I. Based on the previous answer, what is the MOLAR extinction coefficient at max in units of M1cm1 ? SHOW WORK (2 pt)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


