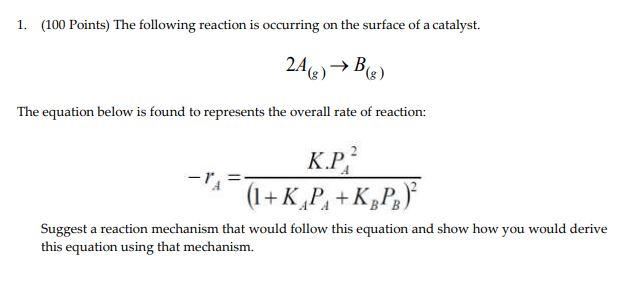
Question: Che 321. Don't give me wrong answers. I will rate it. Thank you. 1. (100 Points) The following reaction is occurring on the surface of
Che 321. Don't give me wrong answers. I will rate it. Thank you.
1. (100 Points) The following reaction is occurring on the surface of a catalyst. 2A(g)B(g) The equation below is found to represents the overall rate of reaction: rA=(1+KAPA+KBPB)2KPA2 Suggest a reaction mechanism that would follow this equation and show how you would derive this equation using that mechanism
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


