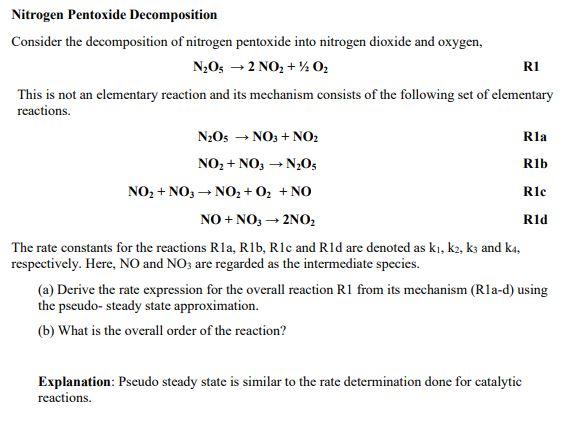
Question: Che 321. Don't give me wrong answers, please. I will rate it. Thank you. N2O52NO2+1/2O2 R1 This is not an elementary reaction and its mechanism
Che 321. Don't give me wrong answers, please. I will rate it. Thank you.
N2O52NO2+1/2O2 R1 This is not an elementary reaction and its mechanism consists of the following set of elementary reactions. N2O5NO3+NO2NO2+NO3N2O5NO2+NO3NO2+O2+NONO+NO32NO2R1aRlbRlcR1d The rate constants for the reactions R1a,R1b,R1c and R1d are denoted as k1,k2,k3 and k4, respectively. Here, NOandNO3 are regarded as the intermediate species. (a) Derive the rate expression for the overall reaction R1 from its mechanism (R1a-d) using the pseudo- steady state approximation. (b) What is the overall order of the reaction? Explanation: Pseudo steady state is similar to the rate determination done for catalytic reactions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


