Question: Che 321. I will rate it with your correct work. Don't give me wrong answers. Thanks. The reaction rate of a 1st-order, elementary catalytic reaction
Che 321. I will rate it with your correct work. Don't give me wrong answers. Thanks.
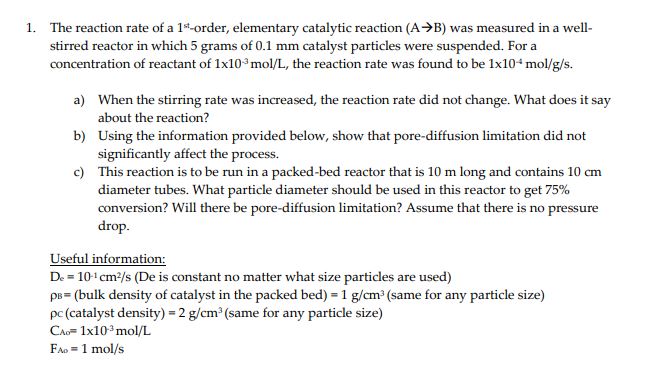
The reaction rate of a 1st-order, elementary catalytic reaction (AB) was measured in a wellstirred reactor in which 5 grams of 0.1mm catalyst particles were suspended. For a concentration of reactant of 1103mol/L, the reaction rate was found to be 1104mol/g/s. a) When the stirring rate was increased, the reaction rate did not change. What does it say about the reaction? b) Using the information provided below, show that pore-diffusion limitation did not significantly affect the process. c) This reaction is to be run in a packed-bed reactor that is 10m long and contains 10cm diameter tubes. What particle diameter should be used in this reactor to get 75% conversion? Will there be pore-diffusion limitation? Assume that there is no pressure drop. Useful information: De=101cm2/s (De is constant no matter what size particles are used) B= (bulk density of catalyst in the packed bed) =1g/cm3 (same for any particle size) c (catalyst density) =2g/cm3 (same for any particle size) CAOA=1103mol/L FA0=1mol/s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


