Question: ChemActivity 5: Resonancu Model 6: More Resonance Structures = Larger Box According to the Electron in a Bax analogy.. - A charge that is localized
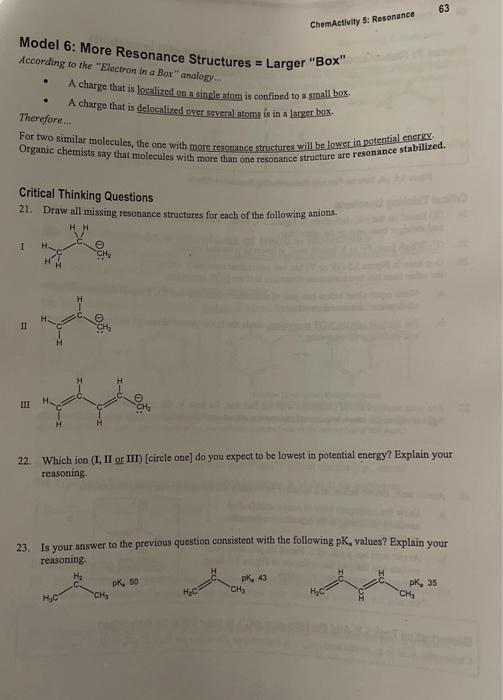
ChemActivity 5: Resonancu Model 6: More Resonance Structures = Larger "Box" According to the "Electron in a Bax" analogy.. - A charge that is localized on a single atom is confined to a groall bor. - A charge that is delocalized over several atoms is in a larger bax. Therefore... For two similar molecules, the one with more resonance structures will be lower in potential energy. Organic chemists say that molecules with more than one resonance strueture are resonance stabillzed. Critical Thinking Questions 21. Draw all missing resonance structures for each of the following anions. I I III 22. Which ion (I, II or III) [circle one] do you expect to be lowest in potential energy? Explain your reasoning. 23. Is your answer to the previous question consistent with the following pK, values? Explain your reasoning
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


