Question: Model 4: Resonance Structures-Important (vs. Second Order r.s.) When you are asked to draw all resonmese structures of an ion you should draw only the
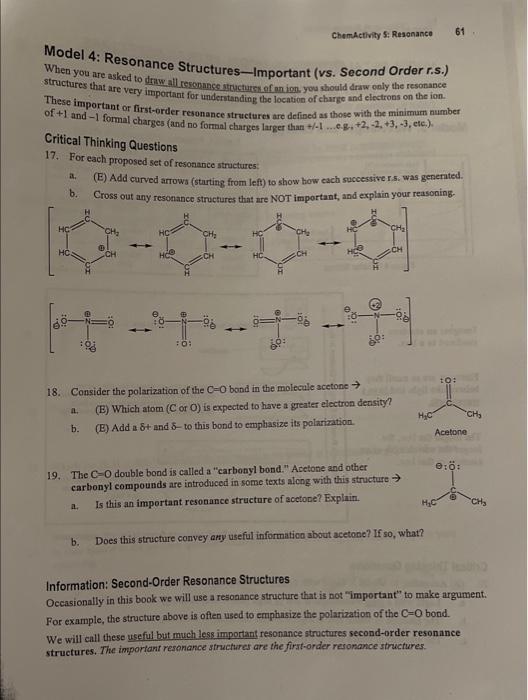
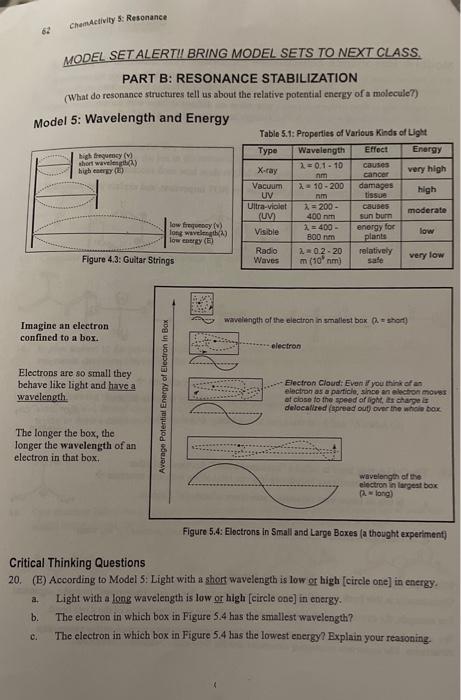
Model 4: Resonance Structures-Important (vs. Second Order r.s.) When you are asked to draw all resonmese structures of an ion you should draw only the resonance structures that are very important for undentanding the location of charge and electrons on the ion. These important or first-order resonance structures are defined as those with the minimum number of +1 and 1 formal charges (and no formal charges larger than +/1...eg. +2,2,+3,3, etc.). Critical Thinking Questions 17. For each proposed set of resonance structures: a. (E) Add curved arrows (starting from left) to show bow cach successive r 5, was genented. b. Cross out any resonance structures that are NOT important, and explain your reasoning. 18. Consider the polarization of the C=O bond in the molecule scetone a. (E) Which atom ( C or O ) is expected to have a greater electron density? b. (E) Add a and - to this bond to emplasize its polarization. 19. The C=O double bond is called a "carbonyl bond." Acetone and other carbonyl compounds are introduced in some texts along with this structure a. Is this an important resonance structure of acetone? Explain. Acotone b. Does this structure convey amy useful information about acetone? If so, what? Information: Second-Order Resonance Structures Occasionally in this book we will use a resonance structure that is not "important" to make argument. For example, the structure above is often used to emphasize the polarization of the C=O bond. We will call these useful byt much less important resonance structures second-order resonance structures. The important resonance structures are the first-order resonance structures. MODEL SET ALERTII BRING MODEL SETS TO NEXT CLASS. PART B: RESONANCE STABILIZATION (What do resonance structures tell us about the relative potential energy of a moleculer) Model 5: Wavelength and Energy Table 5.1: Properties of Various Kinds af Ligtt Imagine an electron confined to a box. Electrons are so small they behave like light and have a wavelength. The longer the box, the longer the wavelength of an electron in that box. Figure 5.4: Eloctrons in Small and Large Boxes (a thought experiment) Critical Thinking Questions 20. (B) According to Model 5: Light with a short wavelength is low or high [circle one] in energy. a. Light with a long wavelength is low or high [circle one] in energy. b. The electron in which box in Figure 5.4 has the smallest wavelength? e. The electron in which box in Figure 5.4 has the lowest energy? Explain your reavoning
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


