Question: - Chemical Reaction Engineering problem - Close to 12.2 billion metric tons of ethylene glycol (EG) were produced in 2000, which ranked it the twenty-sixth
- Chemical Reaction Engineering problem -
Close to 12.2 billion metric tons of ethylene glycol (EG) were produced in 2000, which ranked it the twenty-sixth most produced chemical in the nation that year on a total pound basis. about one-half of the ethylene glycol is used for antifreeze while the other half is used in the manufacture of polyesters. in the polyester category, 88% was used for fibers and 12% for the manufacture of bottles and films. the 2004 selling price for ethylene glycol was $0.28 per pound. it is desired to produce 200 million pounds per year of EG. the reactor is to be operated isothermally. A 1 lb mol/ft^3 solution of ethylene oxide (EO) in water is fed to the reactor (shown in Figure E4-2.1) together with an equal volumetric solution of water containing 0.9 wt% of the catalyst H2SO4. the specific reaction rate constant is 0.311 min^(-1). Practical guidelines for reactor scale-up were recently given by Mukesh and by Warsteel
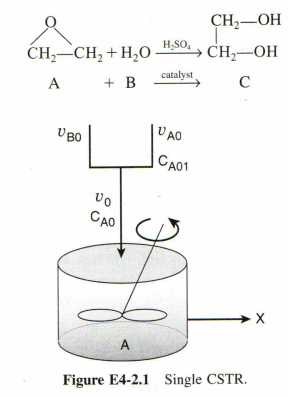
But, consider the following changes made on the conditions (Here, water is in excess compared to ethylene glycol so that its concentration does not change with conversion greatly.
So we can assume the pseudo-first order rate law 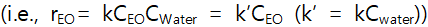 .
.
This is true because 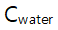 is almost constant through the reaction so that it can be lumped into k).
is almost constant through the reaction so that it can be lumped into k).
(a) Determine the necessary CSTR volume for achieving 50% conversion. (b) Calculate the conversion when three 300-gal reactors were arranged in parallel with equally divided volumetric flow rates. (c) If four 300-gal reactors were arranged in series, calculate the corresponding conversion.
Please upload the graph using Excel
Please write neatly so that I can read it easily
A +Bcatalyst C (k=kCwater)) Cwater
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


