Question: Example 5-2. (1) What conversion would be achieved if three 800-gal CSTRs were placed in series? (2) In parallel with the feed equally divided? (3)
Example 5-2. (1) What conversion would be achieved if three 800-gal CSTRs were placed in series?
(2) In parallel with the feed equally divided?
(3) What are the advantages and disadvantages of adding this third reactor?
(4) FA0 = FA0, that conversion achieved in any one of the reactors will be identical to what would be achieved if the reactor were fed in one stream, FA0 = nFAi0, to one large reactor of volume V = nVi.
Example 5-2
Globally, close to 60 billion pounds of ethylene glycol (EG) were produced in 2016. It previously ranked as the 26th most produced chemical in the nation on a total pound basis. About one-half of the ethylene glycol is used for antifreeze, while the other half is used in the manufacture of polyesters. In the polyester category, 88% was used for fibers and 12% for the manufacture of bottles and films. The 2017 selling price for ethylene glycol was $0.43 per pound. Uses and economics It is desired to produce 200 million pounds per year of EG. The reactor is to be operated isothermally. A 16.1 mol/dm3 solution of ethylene oxide (EO) in water is mixed with an equal volumetric solution of water containing 0.9 wt % of the catalyst H2SO4 and fed to a CSTR. The pseudo first-order specific reactionrate constant is 0.311 min–1, as determined in Example 5-1. Practical guidelines for reactor scale-up are given by Mukesh.
1. If 80% conversion is to be achieved, determine the necessary CSTR volume.
2. If two 800-gal reactors were arranged in parallel with the feed equally divided, what would be the corresponding conversion?
3. If two 800-gal reactors were arranged in series, what would be the corresponding conversion?
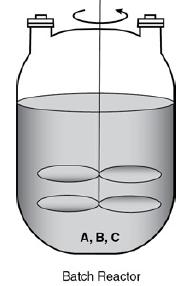
A CSTR consists of an agitator immersed in the liquid. The agitator is rotated in the counter-clockwise direction. There is an inlet through which two reactants A and B enter. For the reactant A, F subscript A0, v subscript A0, and C subscript A 01 are marked. For the reactant B, F subscript B0, v subscript B0, and C subscript B 01 are marked. The product C comes out of the outlet at the bottom of the reactor. The value X and F subscript C are marked for product C.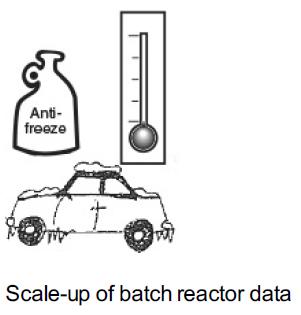
D. Mukesh, Chemical Engineering, 46 (January 2002), www.CHE.com.
Example 5-1
It is desired to design a CSTR to produce 200 million pounds of ethylene glycol per year by hydrolyzing ethylene oxide. However, before the design can be carried out, it is necessary to perform and analyze a batch-reactor experiment to determine the specific reaction-rate constant, k. Because the reaction will be carried out isothermally, the specific reaction rate will need to be determined only at the reaction temperature of the CSTR. At temperatures above 80°C, there is a significant byproduct formation, while at temperatures below 40°C, the reaction does not proceed at a significant rate; consequently, a temperature of 55°C has been chosen. Because water is present in excess, its concentration (55.5 mol/dm3) may be considered constant during the course of the reaction. The reaction is first-order in ethylene oxide.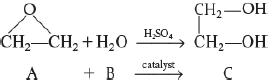
In the laboratory experiment, 500 mL of a 2 M solution (2 kmol/m3) of ethylene oxide
(A) In water was mixed with 500 mL of water
(B) Containing 0.9 wt % sulfuric acid, which is a catalyst. The temperature was maintained at 55°C. The concentration of ethylene glycol
(C) Was recorded as a function of time.
1. Derive an equation for the concentration of ethylene glycol as a function of time.
2. Rearrange the equation derived in (a) to obtain a linear plot of a function concentration versus time.
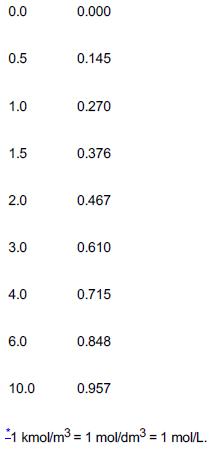
3. Using the data in Table E5-1.1, determine the specific reaction rate, k, at 55°C.
TABLE E5-1.1 CONCENTRATION–TIME DATA
Time (min) Concentration of Ethylene Glycol (C) (kmol/m3)*
Table E5-1.1
Check 10 types of homework problems on the CRE Web site for more solved examples using this algorithm. (http://www.umich.edu/~elements/6e/probsolv/tentypes/index.htm)
FAO UAO CA01 yo CAOI FBO 1 -X, Fc
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
For three CSTRs in series X 1 11 21673 096 For three CSTRs in parall... View full answer

Get step-by-step solutions from verified subject matter experts


