Question: Chemical Reactor Engineering. Please solve step by step. No ChatGPT answers, please. Much appreciated A palladium based monolith reactor is employed for the catalytic oxidation
 Chemical Reactor Engineering. Please solve step by step. No ChatGPT answers, please. Much appreciated
Chemical Reactor Engineering. Please solve step by step. No ChatGPT answers, please. Much appreciated
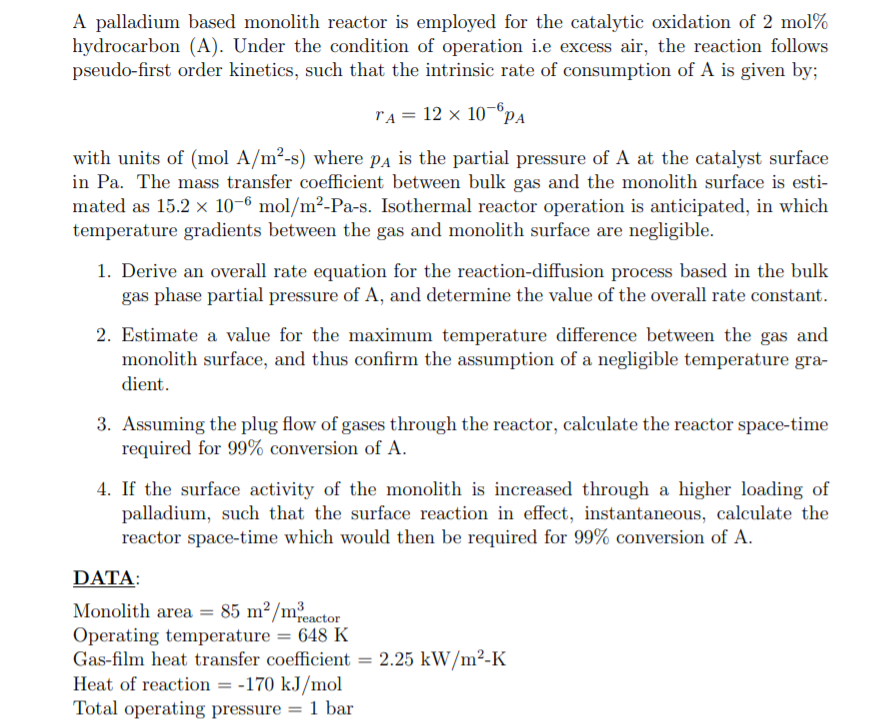
A palladium based monolith reactor is employed for the catalytic oxidation of 2mol% hydrocarbon (A). Under the condition of operation i.e excess air, the reaction follows pseudo-first order kinetics, such that the intrinsic rate of consumption of A is given by; rA=12106pA with units of (molA/m2s) where pA is the partial pressure of A at the catalyst surface in Pa. The mass transfer coefficient between bulk gas and the monolith surface is estimated as 15.2106mol/m2-Pa-s. Isothermal reactor operation is anticipated, in which temperature gradients between the gas and monolith surface are negligible. 1. Derive an overall rate equation for the reaction-diffusion process based in the bulk gas phase partial pressure of A, and determine the value of the overall rate constant. 2. Estimate a value for the maximum temperature difference between the gas and monolith surface, and thus confirm the assumption of a negligible temperature gradient. 3. Assuming the plug flow of gases through the reactor, calculate the reactor space-time required for 99% conversion of A. 4. If the surface activity of the monolith is increased through a higher loading of palladium, such that the surface reaction in effect, instantaneous, calculate the reactor space-time which would then be required for 99% conversion of A. DATA: Monolith area =85m2/mreactor3 Operating temperature =648K Gas-film heat transfer coefficient =2.25kW/m2K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


