Question: Problem statement Chemical reactor engineering - retake Deadline 0 5 / 0 7 / 2 0 2 4 Problem 1 Calculate the reactor volumes for
Problem statement Chemical reactor engineering retake
Deadline
Problem
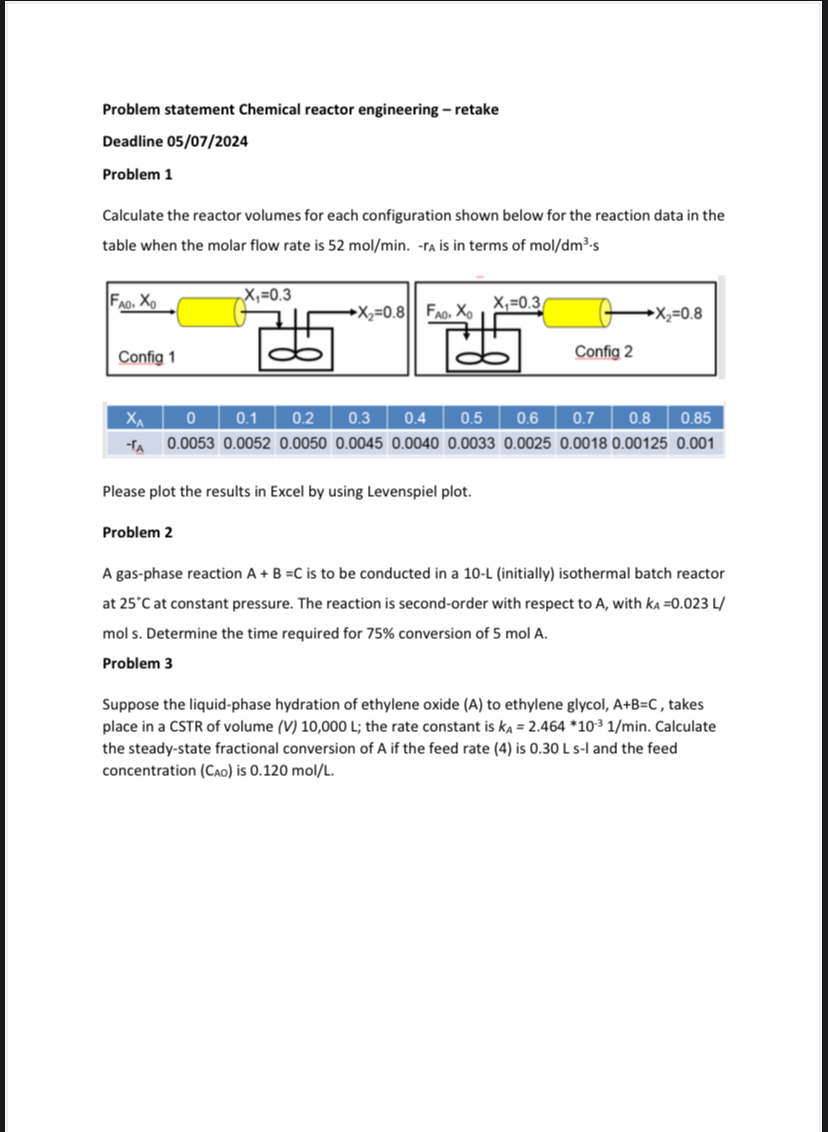
Calculate the reactor volumes for each configuration shown below for the reaction data in the table when the molar flow rate is ra is in terms of
table
Please plot the results in Excel by using Levenspiel plot.
Problem
A gasphase reaction is to be conducted in a initially isothermal batch reactor at at constant pressure. The reaction is secondorder with respect to with mol s Determine the time required for conversion of molA.
Problem
Suppose the liquidphase hydration of ethylene oxide A to ethylene glycol, takes place in a CSTR of volume ; the rate constant is Calculate the steadystate fractional conversion of if the feed rate is I and the feed concentration is
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


