Question: For many gases the temperature (C) dependence of the heat capacity Cp (joules/(g mol) (C))of can be described using this equation: C = a
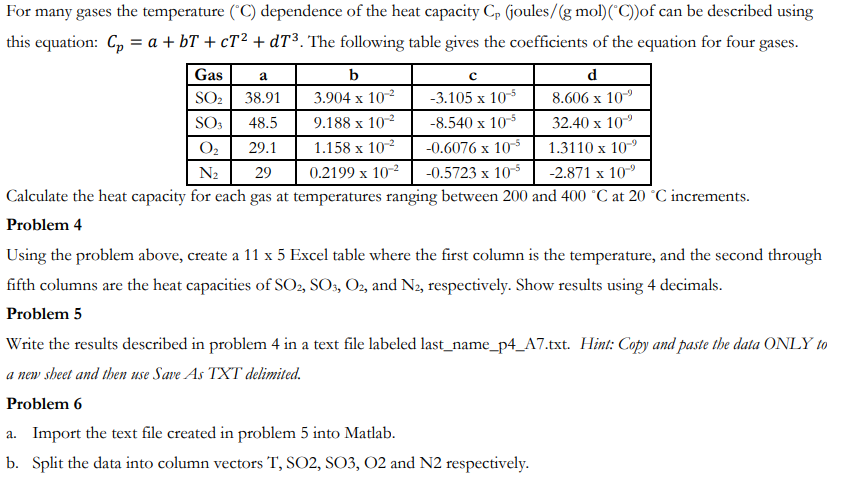
For many gases the temperature (C) dependence of the heat capacity Cp (joules/(g mol) (C))of can be described using this equation: C = a + bT + cT + dT. The following table gives the coefficients of the equation for four gases. Gas a b d SO 38.91 -3.105 x 10-5 3.904 x 10- 9.188 x 10 8.606 x 10 32.40 x 10 SO3 48.5 -8.540 x 10-5 O 29.1 1.158 x 10 -0.6076 x 10-5 1.3110 x 10 N 29 0.2199 x 10- -0.5723 x 10-5 -2.871 x 10 Calculate the heat capacity for each gas at temperatures ranging between 200 and 400 C at 20 C increments. Problem 4 Using the problem above, create a 11 x 5 Excel table where the first column is the temperature, and the second through fifth columns are the heat capacities of SO2, SO3, O2, and N2, respectively. Show results using 4 decimals. Problem 5 Write the results described in problem 4 in a text file labeled last_name_p4_A7.txt. Hint: Copy and paste the data ONLY to a new sheet and then use Save As TXT delimited. Problem 6 a. Import the text file created in problem 5 into Matlab. b. Split the data into column vectors T, SO2, SO3, O2 and N2 respectively.
Step by Step Solution
3.43 Rating (150 Votes )
There are 3 Steps involved in it
HEAT CAPACITY Heat capacity varying with temperature is represented by following equation SPECIFIC HEAT C P a bT cT 2 dT 3 Where T Temperature in C a ... View full answer

Get step-by-step solutions from verified subject matter experts


