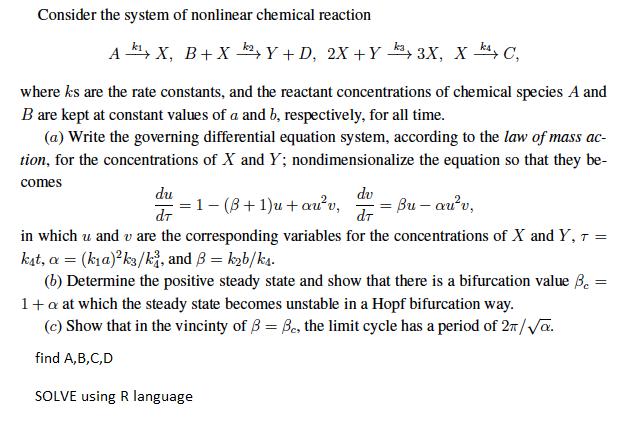
Question: Consider the system of nonlinear chemical reaction A, X, B+Xk2, Y + D, 2X+Y 3X, X4, C, where ks are the rate constants, and
Consider the system of nonlinear chemical reaction A, X, B+Xk2, Y + D, 2X+Y 3X, X4, C, where ks are the rate constants, and the reactant concentrations of chemical species A and B are kept at constant values of a and b, respectively, for all time. (a) Write the governing differential equation system, according to the law of mass ac- tion, for the concentrations of X and Y; nondimensionalize the equation so that they be- comes du Bu-auv, dT in which u and u are the corresponding variables for the concentrations of X and Y, T = kat, a = (ka)ks/ki, and 3= kb/ks. = (b) Determine the positive steady state and show that there is a bifurcation value Be= 1+ a at which the steady state becomes unstable in a Hopf bifurcation way. (c) Show that in the vincinty of B = Be, the limit cycle has a period of 2/a. find A,B,C,D SOLVE using R language = 1-(8+1)u+auv, dv dr =
Step by Step Solution
There are 3 Steps involved in it
Solution Given that ka X ... View full answer

Get step-by-step solutions from verified subject matter experts