Question: Class Open with Google Docs tions.doc oxidation half-reaction: reduction half-reaction: 1. Rank the strength of the following as oxidizing agents, going from strongest to weakest:
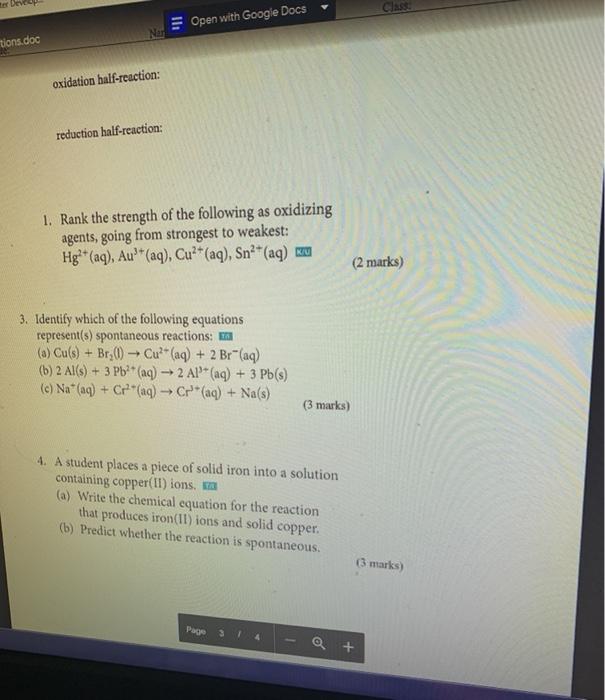
Class Open with Google Docs tions.doc oxidation half-reaction: reduction half-reaction: 1. Rank the strength of the following as oxidizing agents, going from strongest to weakest: Hg+ (aq), Au'* (aq), Cu+ (aq), Sn2+ (aq) ku (2 marks) 3. Identify which of the following equations represent(s) spontaneous reactions: (a) Cu(s) + Br_() Cu?+ (aq) + 2 Br"(aq) (b) 2 Al(s) + 3 Pb2+ (aq) 2 Al!(aq) + 3 Pb(s) (c) Na+ (aq) + C+ (aq) Cr* (aq) + Na(s) (3 marks) 4. A student places a piece of solid iron into a solution containing copper(II)ions sa (a) Write the chemical equation for the reaction that produces iron(11) ions and solid copper. (b) Predict whether the reaction is spontaneous. (3 marks) Page Q +
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


