Question: clear solution, please Problem 6: Among the several solvents that used in the fabrication of solar cells is monochlorobenzol (CHCI). Monochlorobenzol is formed by reaction
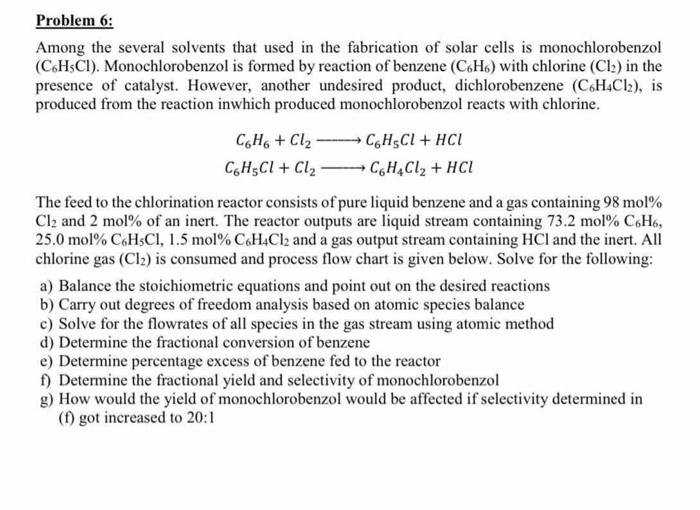
Problem 6: Among the several solvents that used in the fabrication of solar cells is monochlorobenzol (CHCI). Monochlorobenzol is formed by reaction of benzene (C.H.) with chlorine (Cl2) in the presence of catalyst. However, another undesired product, dichlorobenzene (C6H4Cl2), is produced from the reaction inwhich produced monochlorobenzol reacts with chlorine. C6H6 + Cl2 CHECI + HCI CH3Cl + Cl2 CH4Cl2 + HCL The feed to the chlorination reactor consists of pure liquid benzene and a gas containing 98 mol% Cl2 and 2 mol% of an inert. The reactor outputs are liquid stream containing 73.2 mol% C6H6, 25.0 mol% CHCI, 1.5 mol%C6H4Cl2 and a gas output stream containing HCl and the inert. All chlorine gas (Cl2) is consumed and process flow chart is given below. Solve for the following: a) Balance the stoichiometric equations and point out on the desired reactions b) Carry out degrees of freedom analysis based on atomic species balance c) Solve for the flowrates of all species in the gas stream using atomic method d) Determine the fractional conversion of benzene e) Determine percentage excess of benzene fed to the reactor f) Determine the fractional yield and selectivity of monochlorobenzol g) How would the yield of monochlorobenzol would be affected if selectivity determined in (1) got increased to 20:1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


