Question: CO2 C O2 + 1 atom 1 molecule 6.02 x 10 molecules 1 mole 1 molecule 6.02 x 101 molecules 1 mole 6.02 x 1011
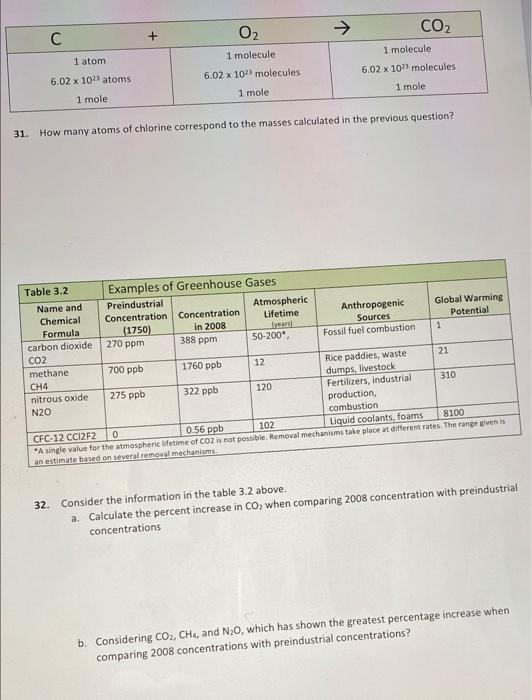
CO2 C O2 + 1 atom 1 molecule 6.02 x 10 molecules 1 mole 1 molecule 6.02 x 101 molecules 1 mole 6.02 x 1011 atoms 1 mole 31. How many atoms of chlorine correspond to the masses calculated in the previous question? Table 3.2 Examples of Greenhouse Gases Name and Preindustrial Atmospheric Chemical Concentration Concentration Global Warming Anthropogenic Lifetime Formula (1750) in 2008 Sources Potential carbon dioxide 270 ppm 388 ppm 50-200 Fossil fuel combustion 1 CO2 methane 700 ppb 1760 ppb 12 Rice paddles, waste 21 dumps, livestock nitrous oxide 275 ppb 322 ppb 120 Fertilizers, industrial 310 N2O production combustion CFC-12 CCI2F2 0 0.56 ppb 102 Liquid coolants, foams 8100 "A single value for the atmospheric lifetime of CO2 is not possible. Removal mechanisms take place at different rates. The range oven is an estimate based on several removal mechanisms CH4 32. Consider the information in the table 3.2 above. a. Calculate the percent increase in Co, when comparing 2008 concentration with preindustrial concentrations b. Considering CO2, CH4, and N20, which has shown the greatest percentage increase when comparing 2008 concentrations with preindustrial concentrations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


