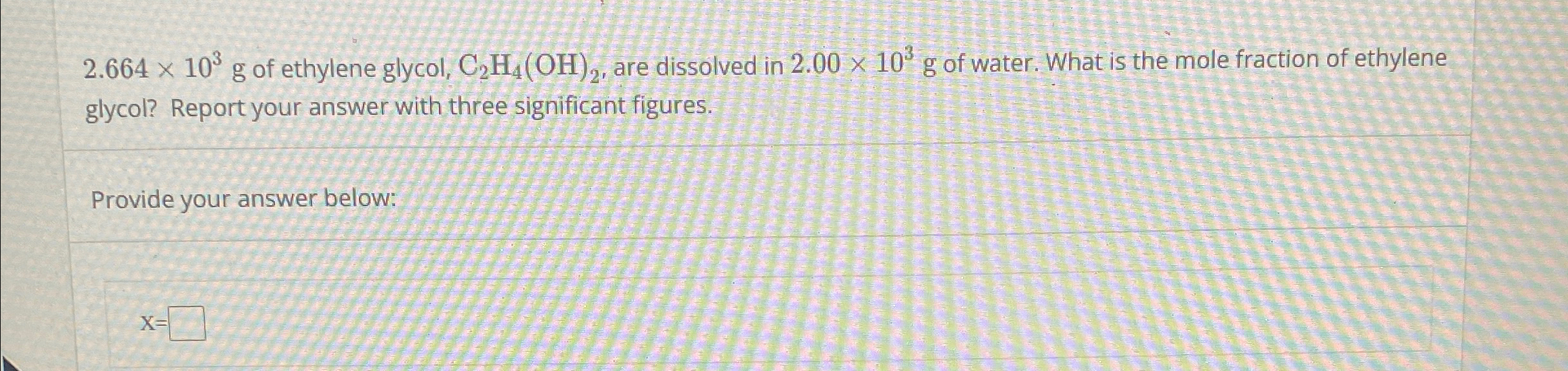
Question: code class = asciimath > 2 . 6 6 4 times 1 0 ^ ( 3 ) g of ethylene glycol, C 2 H
code class"asciimath"times g of ethylene glycol, are dissolved in of water. What is the mole fraction of ethylene glycol? Report your answer with three significant figures. Provide your answer below:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


