Question: Columns in the periodic table are called groups, and the rows are called periods. Notice that the groups are labeled 1 to 18 and
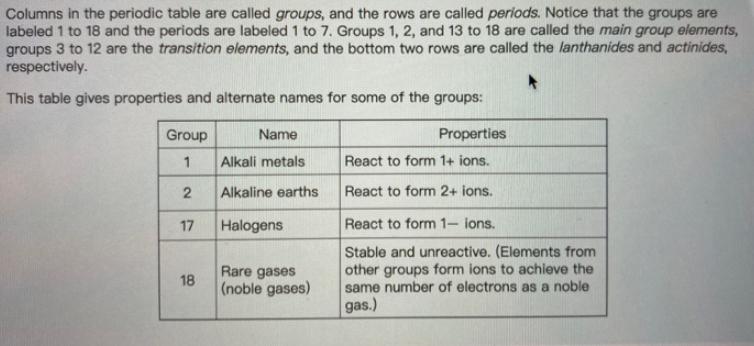
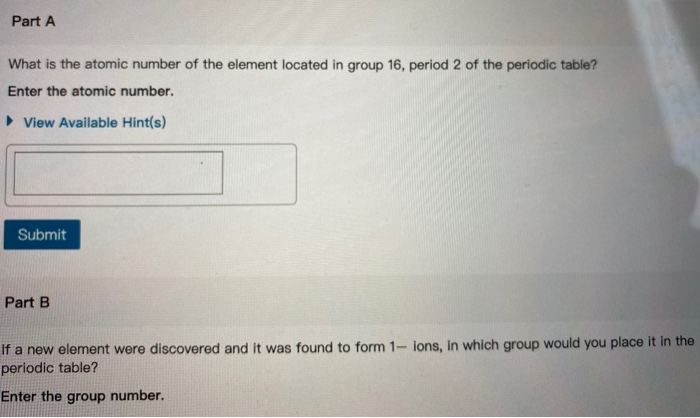
Columns in the periodic table are called groups, and the rows are called periods. Notice that the groups are labeled 1 to 18 and the periods are labeled 1 to 7. Groups 1, 2, and 13 to 18 are called the main group elements, groups 3 to 12 are the transition elements, and the bottom two rows are called the lanthanides and actinides, respectively. This table gives properties and alternate names for some of the groups: Properties Group 1 Name Alkali metals React to form 1+ ions. 2 Alkaline earths React to form 2+ ions. 17 Halogens 18 18 Rare gases (noble gases) React to form 1- ions. Stable and unreactive. (Elements from other groups form ions to achieve the same number of electrons as a noble gas.) Part A What is the atomic number of the element located in group 16, period 2 of the periodic table? Enter the atomic number. View Available Hint(s) Submit Part B If a new element were discovered and it was found to form 1- ions, in which group would you place it in the periodic table? Enter the group number.
Step by Step Solution
3.38 Rating (145 Votes )
There are 3 Steps involved in it
Part A The atomic number of the elemen... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
663dfa8a486d5_960695.pdf
180 KBs PDF File
663dfa8a486d5_960695.docx
120 KBs Word File


