Question: Complete the statements about the redox reaction below. 3 electrolytic Cu+(aq) 1 5 2 2Cu+ (aq) + Fe(s) 2Cu*(aq) + Fe+ (aq) Half-reactions: Fe+
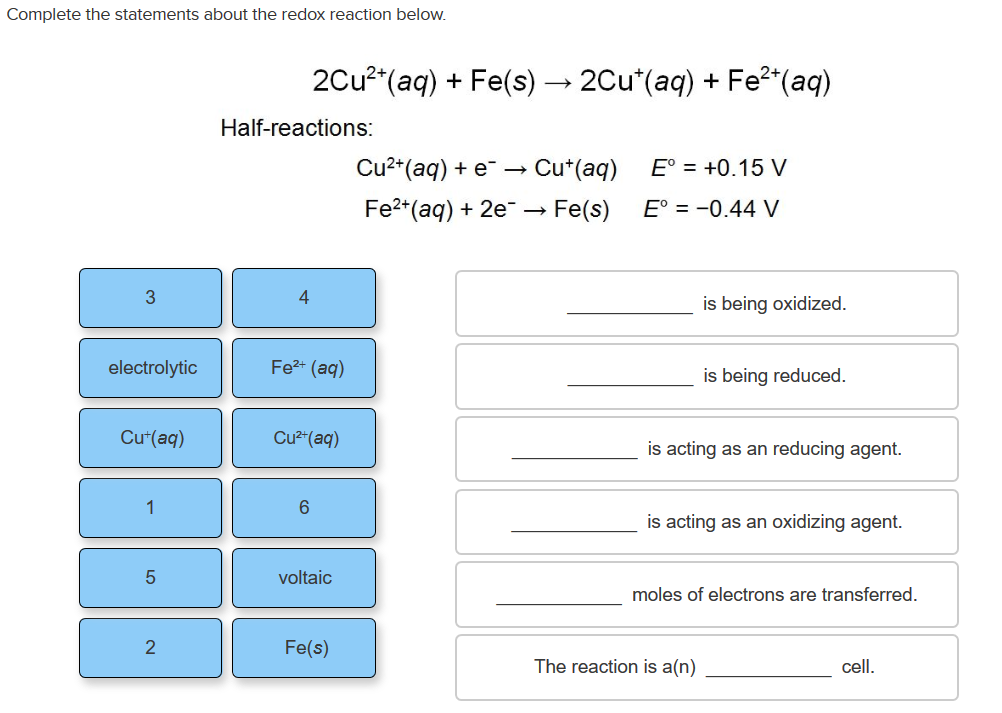
Complete the statements about the redox reaction below. 3 electrolytic Cu+(aq) 1 5 2 2Cu+ (aq) + Fe(s) 2Cu*(aq) + Fe+ (aq) Half-reactions: Fe+ (aq) Cu+ (aq) 6 voltaic Fe(s) Cu+ (aq) + e- Fe+ (aq) + 2e -> Cu+ (aq) Fe(s) E = +0.15 V E = -0.44 V is being oxidized. is being reduced. is acting as an reducing agent. is acting as an oxidizing agent. The reaction is a(n) moles of electrons are transferred. cell.
Step by Step Solution
3.41 Rating (151 Votes )
There are 3 Steps involved in it
Consider overall reaction 2 Cu 2 aq Fe s ightarrow 2 Cu aq Fe aq 2 In above reaction oxidation state ... View full answer

Get step-by-step solutions from verified subject matter experts


