Question: Please explain your answer. Each cyclic structure shown below contains a heteroatom that has lone-pair electrons. For each structure, answer the following questions: Are the
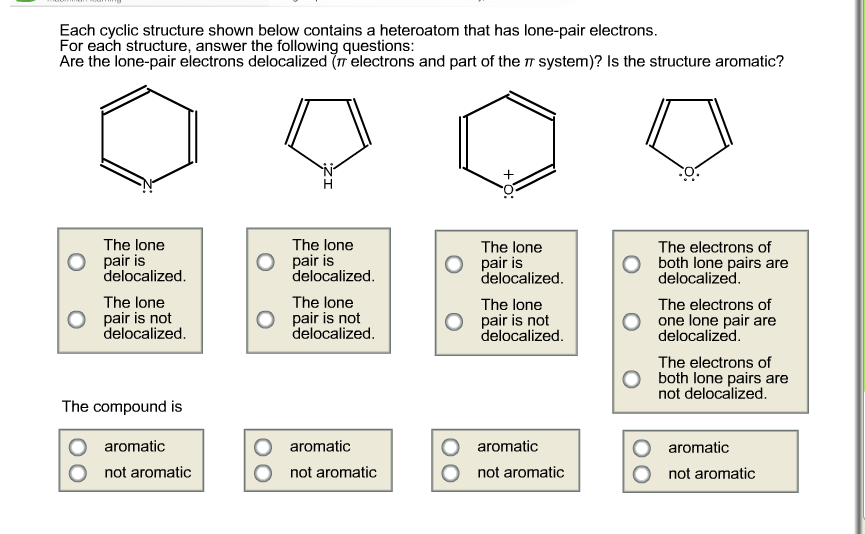
Please explain your answer.
Each cyclic structure shown below contains a heteroatom that has lone-pair electrons. For each structure, answer the following questions: Are the lone-pair electrons delocalized ( electrons and part of the system)? Is the structure aromatic? The lone pair is delocalized. The lone pair is not delocalized. The compound is aromatic not aromatic The lone pair is delocalized. The lone pair is not delocalized. aromatic not aromatic +O: The lone pair is delocalized. The lone pair is not delocalized. aromatic not aromatic The electrons of both lone pairs are delocalized. The electrons of one lone pair are delocalized. The electrons of both lone pairs are not delocalized. aromatic not aromatic
Step by Step Solution
3.46 Rating (159 Votes )
There are 3 Steps involved in it
The lone pair not de localised is The compound is ... View full answer

Get step-by-step solutions from verified subject matter experts


