Question: Compound B has the formula M S. A 20.00 ml. portion of 1938 M HCl was added to 0.8393 g of Compound B with following
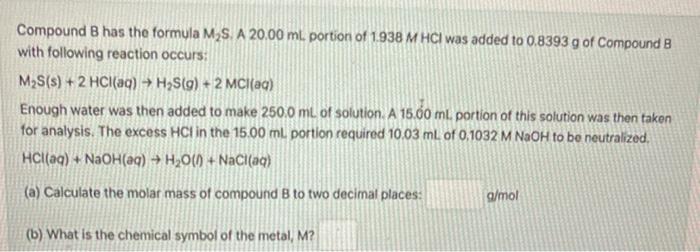
Compound B has the formula M S. A 20.00 ml. portion of 1938 M HCl was added to 0.8393 g of Compound B with following reaction occurs: M2S(s) + 2 HCl(aq) H2S(9) + 2 MCl(aq) Enough water was then added to make 250,0 mL of solution. A 15.00 ml. portion of this solution was then taken for analysis. The excess HCl in the 15.00 ml portion required 10.03 mL of 0.1032 M NaOH to be neutralized, HCl(aq) + NaOH(aq) + H2O(1) + NaCl(aq) (a) Calculate the molar mass of compound B to two decimal places: g/mol (b) What is the chemical symbol of the metal, M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


