Question: Compounds A and B react to give a single product, C. From the choices below, choose the correct rate law and units of the rate
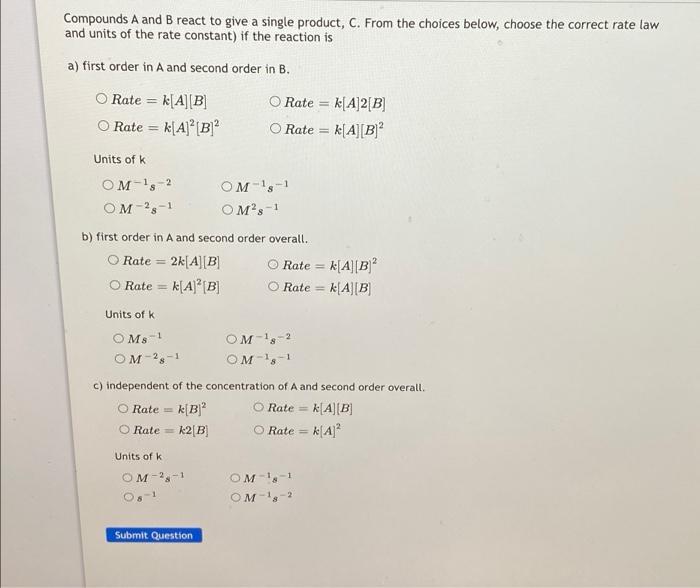
Compounds A and B react to give a single product, C. From the choices below, choose the correct rate law and units of the rate constant) if the reaction is a) first order in A and second order in B. Rate = k[AB Rate = k[A] [B] O Rate = k[A]2[B] Rate = k[A][B] Units of k OM-15-2 OM-28-1 OM-1,-1 OM's-1 b) first order in A and second order overall. Rate = 2k[A][B O Rate = k[A][B) Rate = k[A][B] Rate = k[A][B] Units of K OMS! OM-18-2 OM-28-1 OM-1,-1 c) independent of the concentration of A and second order overall O Rate k[B) Rate = k[A][B] O Rate = k2[B O Rate KA Units of k OM-2.-1 OM 1-1 OM-10-2 Submit
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


