Question: Computer Project Spring, 2023 Due: Thursday, April 20, 2023 Note: You may work in teams of no more than four people. The following experimentally determined
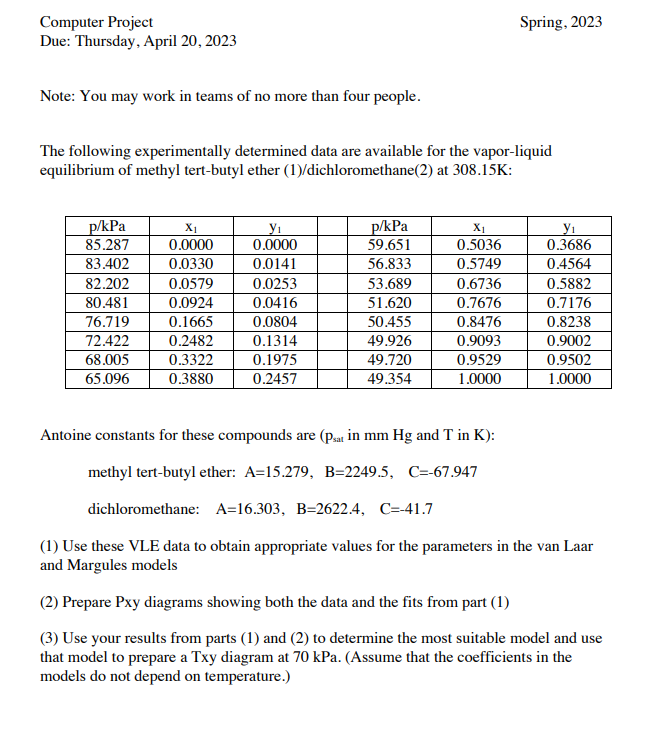
Computer Project Spring, 2023 Due: Thursday, April 20, 2023 Note: You may work in teams of no more than four people. The following experimentally determined data are available for the vapor-liquid equilibrium of methyl tert-butyl ether (1)/dichloromethane(2) at 308.15K : Antoine constants for these compounds are ( psat in mmHg and T in K ): methyl tert-butyl ether: A=15.279,B=2249.5,C=67.947 dichloromethane: A=16.303,B=2622.4,C=41.7 (1) Use these VLE data to obtain appropriate values for the parameters in the van Laar and Margules models (2) Prepare Pxy diagrams showing both the data and the fits from part (1) (3) Use your results from parts (1) and (2) to determine the most suitable model and use that model to prepare a Txy diagram at 70kPa. (Assume that the coefficients in the models do not depend on temperature.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


