Question: Consider a buffer solution consisting of CH3NH3Cl and CH3NH2. Which of the following statements are true concerning this solution? (KaforCH3NH3+=2.31011). If NaOH were added to
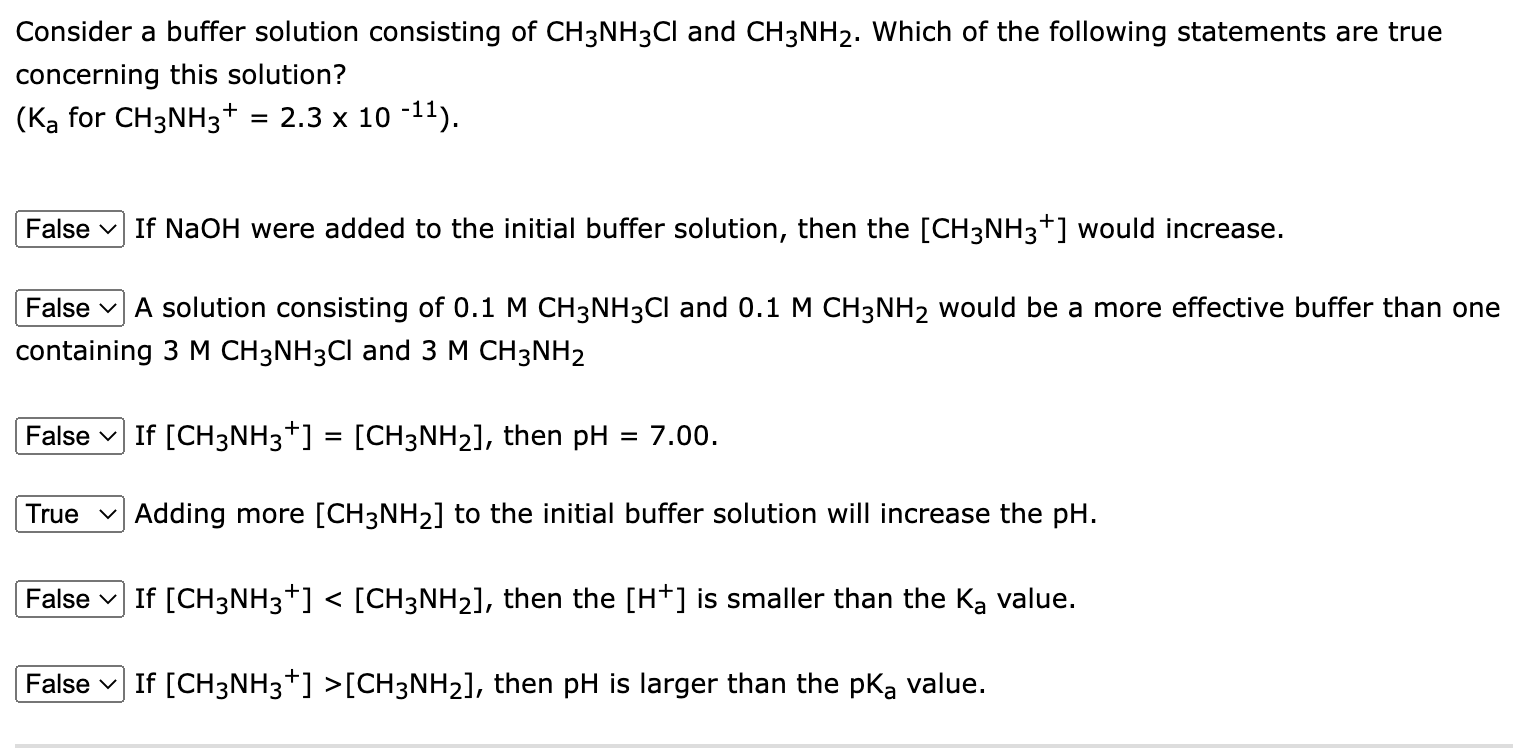
Consider a buffer solution consisting of CH3NH3Cl and CH3NH2. Which of the following statements are true concerning this solution? (KaforCH3NH3+=2.31011). If NaOH were added to the initial buffer solution, then the [CH3NH3+]would increase. A solution consisting of 0.1MCH3NH3Cl and 0.1MCH3NH2 would be a more effective buffer than one containing 3MCH3NH3Cl and 3MCH3NH2 If [CH3NH3+]=[CH3NH2], then pH=7.00. Adding more [CH3NH2] to the initial buffer solution will increase the pH. If [CH3NH3+][CH3NH2], then pH is larger than the pKa value
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


