Question: 6.5(a) The following temperature/composition data were obtained for a mixture of octane (O) and methylbenzene (M) at 1.00atm, where x is the mole traction in
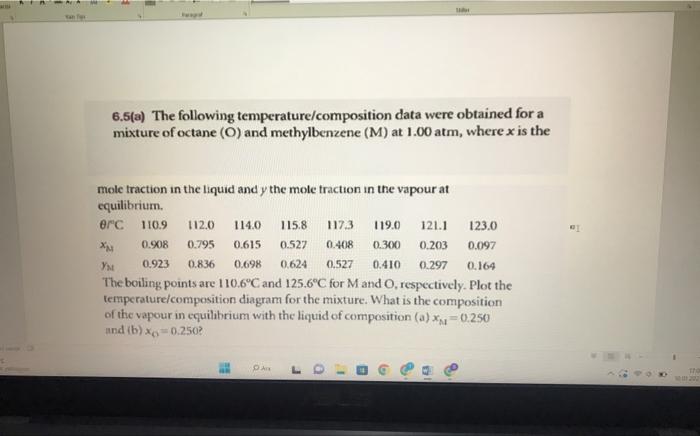
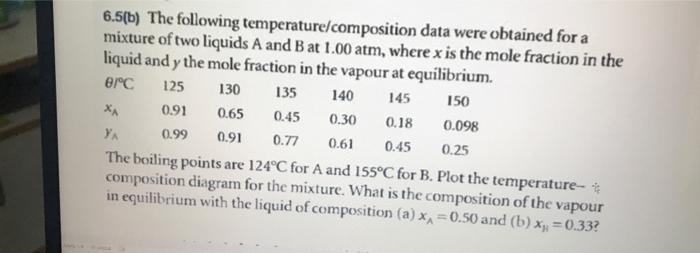
6.5(a) The following temperature/composition data were obtained for a mixture of octane (O) and methylbenzene (M) at 1.00atm, where x is the mole traction in the liquid and y the mole traction in the vapour at equilibrium. The beiling points are 110.6C and 125.6C for M and O, respectively. Plot the temperature/composition diagram for the mixture. What is the composition of the vapour in equilibrium with the liquid of composition (a) xM=0.250 and (b) x0=0.250 ? 6.5(b) The following temperature/composition data were obtained for a mixture of two liquids A and B at 1.00atm, where x is the mole fraction in the liquid and y the mole fraction in the vannwen....as.. the oouing points are 124C for A and 155C for B. Plot the temperature- it composition diagram for the mixture. What is the composition of the vapour in equilibrium with the liquid of composition (a) xA=0.50 and (b) xH=0.33
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


