Question: Consider a constant pressure batch reactor in which a reversible liquid phase reaction occurs. The reaction is: k A+B= C+D where: (-1) = *C. (
Consider a constant pressure batch reactor in which a reversible liquid phase reaction occurs. The reaction is:
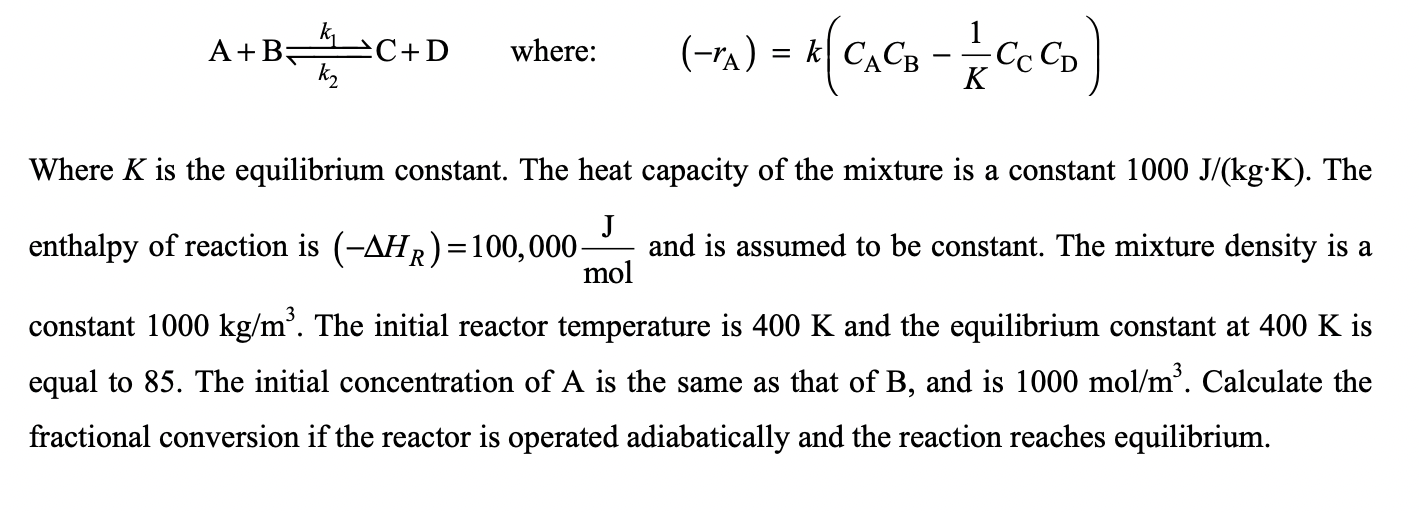
k A+B= C+D where: (-1) = *C. ( = K Where K is the equilibrium constant. The heat capacity of the mixture is a constant 1000 J/(kg:K). The J enthalpy of reaction is (-AHR)=100,000 and is assumed to be constant. The mixture density is a mol constant 1000 kg/m. The initial reactor temperature is 400 K and the equilibrium constant at 400 K is equal to 85. The initial concentration of A is the same as that of B, and is 1000 mol/m. Calculate the fractional conversion if the reactor is operated adiabatically and the reaction reaches equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


