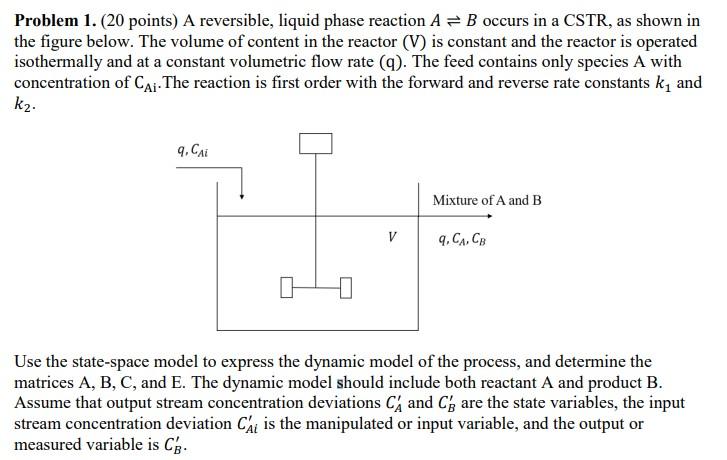
Question: Please explain step by step. Thank you Problem 1. (20 points) A reversible, liquid phase reaction A = B occurs in a CSTR, as shown
Please explain step by step. Thank you
Problem 1. (20 points) A reversible, liquid phase reaction A = B occurs in a CSTR, as shown in the figure below. The volume of content in the reactor (V) is constant and the reactor is operated isothermally and at a constant volumetric flow rate (9). The feed contains only species A with concentration of Cai The reaction is first order with the forward and reverse rate constants ky and k2. q, Cai Mixture of A and B V 9.CCB Use the state-space model to express the dynamic model of the process, and determine the matrices A, B, C, and E. The dynamic model should include both reactant A and product B. Assume that output stream concentration deviations C and C are the state variables, the input stream concentration deviation Chi is the manipulated or input variable, and the output or measured variable is Cg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


