Question: Consider a CSTR in which a liquid-phase reaction: R - Products, is being carried out. The inlet volumetric flow rate vo is 10 L/min. The
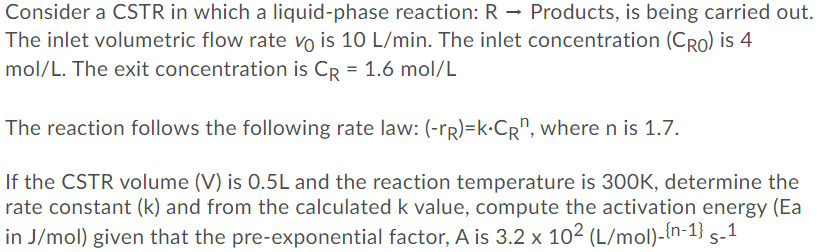
Consider a CSTR in which a liquid-phase reaction: R - Products, is being carried out. The inlet volumetric flow rate vo is 10 L/min. The inlet concentration (Cro) is 4 mol/L. The exit concentration is CR = 1.6 mol/L The reaction follows the following rate law: (-PR)=k.CR", where n is 1.7. If the CSTR volume (V) is 0.5L and the reaction temperature is 300K, determine the rate constant (k) and from the calculated k value, compute the activation energy (Ea in J/mol) given that the pre-exponential factor, A is 3.2 x 102 (L/mol)-{n-1} s-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


